0823
Evaluation of primary and secondary collateral pathways in carotid artery stenosis patients before and after revascularization therapy1School of Medicine, Department of Neuroradiology, Technical University of Munich, Munich, Germany, 2Department of Neurology, Goethe University Frankfurt, Frankfurt, Germany, 3School of Medicine, Clinic of Neurology, Technical University of Munich, Munich, Germany, 4Philips Healthcare, Hamburg, Germany, 5MRRC, Yale University, New Haven, CT, United States
Synopsis
Internal carotid artery stenosis (ICAS) is a well-known risk factor for stroke. While collaterals play a pivotal role in ischemic stroke, their effects in ICAS are not entirely understood. We assessed primary collaterals, i.e., configuration of the Circle of Willis by MR-Angiography, and high Coefficients of Variance in Dynamic Susceptibility Contrast MRI as surrogate of secondary collaterals. Moreover, individual watershed areas (iWSA) were determined in a group of ICAS patients and healthy volunteers. Our results demonstrate post-interventional shifts of iWSA, mainly influenced by primary collateral flow. No secondary collateral flow was found in patients similar to age-matched healthy participants.
Introduction
Internal carotid artery stenosis (ICAS) is a known risk factor for stroke1,2 and can lead to cognitive decline.3,4 The actual extent of hemodynamic impairment, however, is difficult to determine from stenosis degree alone.5 Especially individual collateral blood flow might maintain cerebral hemodynamics to a certain extent.6,7 Collaterals can be distinguished in primary collaterals, that are part of the Circle of Willis (CoW), and secondary collaterals, including leptomeningeal vessels.7 While it is known that collaterals can affect the outcome in ischemic stroke,9–11 their role in chronic hypoperfusion is not completely understood. This is partly due to the fact, that especially imaging of secondary collaterals is challenging and often based on invasive methods.8,12The CoW and, in consequence, primary collateral flow can be evaluated using MR-Angiography. A promising clinically applicable approach to identify secondary collaterals is based on coefficients of variance (CoV) analysis of dynamic susceptibility contrast (DSC) MRI.8 High CoV was recently proposed as a surrogate marker for pial collaterals.8
In addition, individual watershed areas (iWSA) may be used to assess disease progression in ICAS patients. Located at the edges of vascular territories, they are common locations of hypoperfusion-related infarcts.1,13–15 The sensitivity of these regions to hemodynamic alterations is based on their location at the junction of vascular territories.2 Spatial shifts of iWSAs have been previously reported in ICAS patients16,17 and their spatial normalization might be an indicator of treatment success.
In a prospective study we aimed to assess the relation between primary and secondary collaterals as well as the spatial extent of iWSA in a cohort of ICAS patients before and after revascularization treatment in comparison with age-matched healthy controls.
Methods
Seventeen healthy controls (mean age at baseline scan: 70.7±5.4 y) and 16 patients (mean age at baseline scan: 71.9±6.1 y, see Tab.1) with clinically asymptomatic unilateral high grade ICAS (NASCET18 >70% ) underwent MRI twice (mean time between baseline scan 1 and follow-up scan 2: 14.6±4.6 months) on a 3T Philips Ingenia (Philips Healthcare, Best, Netherlands) using a 32-channel head-coil. Imaging protocol and parameters are shown in Figure 1. ICAS patients received revascularization treatment between both scans by either carotid endarterectomy (CEA, n=6) or carotid artery stenting (CAS, n=10).Based on a method developed by Kaczmarz et al.,19 individual WSAs were determined for each subject and scan (Fig.2B). Spatial congruency of iWSAs at baseline and follow-up was assessed by Dice Coefficients (DC). Every participant’s primary collateralization was rated complete or incomplete by the CoW, meaning at least one atretic anterior or posterior communicating artery (Table 1). From the DSC time-series, CoV was calculated for each voxel, thresholding at 70th percentile with removal of large vessels and ventricles.8 Within this high CoV-mask, quantitative CoV values were extracted for each participant’s hemisphere and two-sample t-tests applied.
Results
Exemplary data is shown in Figure 2. Overall, patients revealed significantly lower iWSA congruency in the hemisphere ipsilateral to the stenosis compared to the contralateral side (mean DC ipsilateral/contralateral to the stenosis: 0.59/0.64, p=0.03). Patients with an incomplete CoW configuration presented a significantly reduced iWSA overlap in the hemisphere ipsilateral to the stenosis vs. the contralateral side (DC ipsi/contra 0.56/0.65 p=0.007), indicating treatment-related shift of iWSAs (Fig. 3). In patients with complete CoW configuration, no differences between hemispheres were detected (p=0.94). In controls, iWSAs were stable over time (mean DC left/right hemisphere: 0.63/0.62, p=0.83). Secondary collateralization, assessed by CoV values within the high CoV mask, remained stable across hemispheres in both groups for both scan times (Fig.4).Discussion
We successfully analyzed primary and secondary collaterals and their effects in our cohort of ICAS patients. Revascularization treatment led to shifts of iWSA in patients with incomplete CoW configuration. This suggests that ICAS might impair cerebral hemodynamics more in this subgroup, strengthening the indication for stenosis treatment. Higher overlap of pre- and post-treatment iWSA in patients with complete CoW indicates higher stability of flow territories and therefore a protective effect of primary collaterals, which is in accordance with literature.20–22The analysis of CoV revealed stable values in both groups, no differences between hemispheres and was very similar in ICAS patients and healthy controls (Fig.3). Thus, secondary collaterals do not seem to play a major role in our cohort of asymptomatic ICAS patients. While pial collaterals in ICAS patients have been reported,23 others did not find evidence of secondary collateral flow.24,25 Those differences are most likely related to differences in the examined patient collective.23 Thus, pial collaterals may be associated with more severe hemodynamic compromise,26,27 which might not be given in this cohort of patients.
Conclusion
In our longitudinal study, we considered primary and secondary collaterals as well as iWSA to investigate the hemodynamic impact of ICAS in asymptomatic patients. After revascularization treatment, iWSA location was altered in patients with incomplete CoW, while we found no evidence of secondary collateral flow in patients compared to the healthy control group. Further investigation of collateral pathways is highly promising to deepen our understanding of underlying pathophysiological mechanisms and optimized outcome in these patients.Acknowledgements
No acknowledgement found.References
1. Momjian-Mayor I, Baron J-C. The Pathophysiology of Watershed Infarction in Internal Carotid Artery Disease: Review of Cerebral Perfusion Studies. Stroke 2005; 36: 567–577.
2. D’Amore C, Paciaroni M. Border-Zone and Watershed Infarctions. In: Paciaroni M, Agnelli G, Caso V, et al. (eds) Frontiers of Neurology and Neuroscience. Basel: KARGER, pp. 181–184.
3. Balestrini S, Perozzi C, Altamura C, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology 2013; 80: 2145–2150.
4. Göttler J, Kaczmarz S, Nuttall R, et al. The stronger one-sided relative hypoperfusion, the more pronounced ipsilateral spatial attentional bias in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab2020; 40: 314–327.
5. Powers WJ. The Effect of Hemodynamically Significant Carotid Artery Disease on the Hemodynamic Status of the Cerebral Circulation. Ann Intern Med 1987; 106: 27.
6. Brozici M, van der Zwan A, Hillen B. Anatomy and Functionality of Leptomeningeal Anastomoses: A Review. Stroke 2003; 34: 2750–2762.
7. Liebeskind DS. Collateral Circulation. Stroke 2003; 34: 2279–2284.
8. Seiler A, Lauer A, Deichmann R, et al. Signal variance-based collateral index in DSC perfusion: A novel method to assess leptomeningeal collateralization in acute ischaemic stroke. J Cereb Blood Flow Metab 2020; 40: 574–587.
9. Lima FO, Furie KL, Silva GS, et al. The Pattern of Leptomeningeal Collaterals on CT Angiography Is a Strong Predictor of Long-Term Functional Outcome in Stroke Patients With Large Vessel Intracranial Occlusion. Stroke2010; 41: 2316–2322.
10. Kim SJ, Son JP, Ryoo S, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke: Collateral Flow Imaging. Ann Neurol 2014; 76: 356–369.
11. Villringer K, Zimny S, Galinovic I, et al. The Association Between Recanalization, Collateral Flow, and Reperfusion in Acute Stroke Patients: A Dynamic Susceptibility Contrast MRI Study. Front Neurol 2019; 10: 1147.
12. McVerry F, Liebeskind DS, Muir KW. Systematic Review of Methods for Assessing Leptomeningeal Collateral Flow. AJNR Am J Neuroradiol 2012; 33: 576–582.
13. Del Sette M, Eliasziw M, Streifler JY, et al. Internal Borderzone Infarction: A Marker for Severe Stenosis in Patients With Symptomatic Internal Carotid Artery Disease. Stroke 2000; 31: 631–636.
14. Schröder J, Heinze M, Günther M, et al. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. NeuroImage: Clinical 2019; 22: 101779.
15. Kaczmarz S, Göttler J, Petr J, et al. Hemodynamic impairments within individual watershed areas in asymptomatic carotid artery stenosis by multimodal MRI. J Cereb Blood Flow Metab 2020.
16. van Laar PJ, Hendrikse J, Klijn CJM, et al. Symptomatic Carotid Artery Occlusion: Flow Territories of Major Brain-Feeding Arteries. Radiology 2007; 242: 526–534.
17. Richter V, Helle M, van Osch MJP, et al. MR Imaging of Individual Perfusion Reorganization Using Superselective Pseudocontinuous Arterial Spin-Labeling in Patients with Complex Extracranial Steno-Occlusive Disease. AJNR Am J Neuroradiol 2017; 38: 703–711.
18. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991; 22: 711–720.
19. Kaczmarz S, Griese V, Preibisch C, et al. Increased variability of watershed areas in patients with high-grade carotid stenosis. Neuroradiology 2018; 60: 311–323.
20. Henderson RD, Eliasziw M, Fox AJ, et al. Angiographically Defined Collateral Circulation and Risk of Stroke in Patients With Severe Carotid Artery Stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31(1):128-132.
21. Bae YJ, Jung C, Kim JH, et al. Quantitative Magnetic Resonance Angiography in Internal Carotid Artery Occlusion with Primary Collateral Pathway. J Stroke 2015; 17: 320–326.
22. Kimmel ER, Al Kasab S, Harvey JB, et al. Absence of Collaterals is Associated with Larger Infarct Volume and Worse Outcome in Patients with Large Vessel Occlusion and Mild Symptoms. Journal of Stroke and Cerebrovascular Diseases 2019; 28: 1987–1992.
23. Seiler A, Brandhofe A, Gracien R-M, et al. DSC perfusion-based collateral imaging and quantitative T2 mapping to assess regional recruitment of leptomeningeal collaterals and microstructural cortical tissue damage in unilateral steno-occlusive vasculopathy. J Cereb Blood Flow Metab 2020.
24. Dankbaar JW, Kerckhoffs KGP, Horsch AD, et al. Internal Carotid Artery Stenosis and Collateral Recruitment in Stroke Patients. Clin Neuroradiol 2018; 28: 339–344.
25. Hartkamp NS, Petersen ET, Chappell MA, et al. Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. J Cereb Blood Flow Metab 2018; 38: 2021–2032.
26. Roach BA, Donahue MJ, Davis LT, et al. Interrogating the Functional Correlates of Collateralization in Patients with Intracranial Stenosis Using Multimodal Hemodynamic Imaging. AJNR Am J Neuroradiol 2016; 37: 1132–1138.
27. Hartkamp NS, Hendrikse J, de Borst GJ, et al. Intracerebral steal phenomenon in symptomatic carotid artery disease. Journal of Neuroradiology 2019; 46: 173–178.
Figures
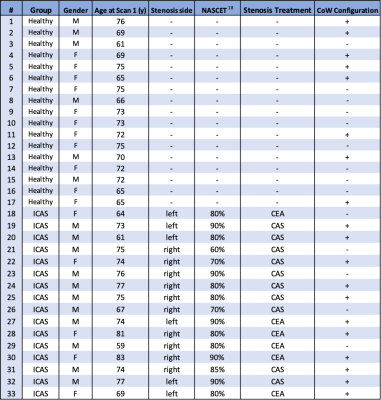
Table 1. Overview on study participants, i.e. healthy volunteers and ICAS patients.
Demographic data comprise gender and age at baseline scan in years and disease related information regarding the side and severity of the stenosis and type of treatment (carotid endarterectomy (CEA) or carotid artery stenting (CAS)). Additionally, configuration of the Circle of Willis (CoW) was dichotomized into good(+) and poor(-) by two experienced neuroradiologists in consensus.
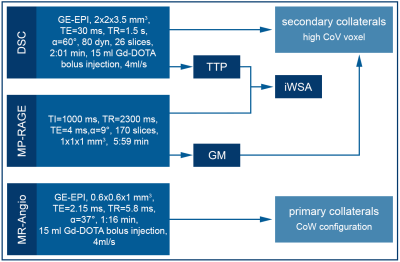
Figure 1. MRI Protocol and Processing.
The scan protocol included for both scans structural MRI (MP-Rage) as well as MR Angiography to determine the configuration of the Circle of Willis (CoW) and DSC MRI. Voxels with high Coefficient of Variance (CoV) were calculated from the DSC time series and evaluated within grey matter (GM). In addition, iWSA were semi-automatically segmented based on DSC-derived Time To Peak (TTP) maps.
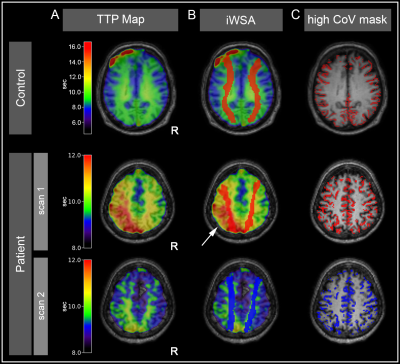
Figure 2 - Exemplary data of a healthy volunteer and an ICAS patient.
Data of a healthy participant are shown in the first row: (A) Time to Peak (TTP) map used for (B) individual watershed area (iWSA) segmentation. (C) High CoV mask as a surrogate for pial collaterals. Comparing data from a patient with left-sided ICAS before (2nd row) and after treatment (3rd row), demonstrates reduction of ipsilaterally elevated TTP (arrow) and shift of iWSA between before (red) and after (blue) treatment, while the CoV-mask remained stable.

Figure 3. Box plots of DC indicate stronger spatial shifts of iWSAs in patients with poor Circle of Willis configuration.
The boxes contain all single-subject DC values between the first and third quartiles, the line inside marks the median, and the whiskers reach from minimum to maximum (not including outliers). In patients with poor Circle of Willis configuration (left), iWSA overlap between scan 1 and 2 differed more between hemispheres (p=0.007) while patients with sound configuration of the CoW did not present significant side differences (p=0.938).
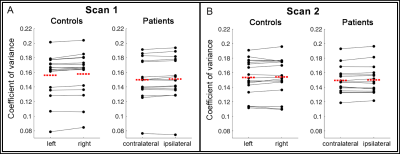
Figure 4. Paired Scatter Plots comparing single-subject coefficients of variance within the high CoV mask.
Data points represent mean coefficients of variance (CoV) of each subject for each hemisphere, lines connect the mean CoV between hemispheres. The red dashed line displays the group average. Stable CoV was found between hemispheres for both groups and scans. A two-sided t-test revealed no significant differences between hemispheres (controls in scan 1/2: p=0.86/0.94; patients in scan 1/2: p=0.92/0.92).