0805
Evaluation of Deep-Learning Reconstructed High-Resolution 3D Lumbar Spine MRI to Improve Image Quality
Simon Sun1, Ek Tsoon Tan1, John A Carrino1, Douglas Nelson Mintz1, Meghan Sahr1, Yoshimi Endo1, Edward Yoon1, Bin Lin1, Robert M Lebel2, Suryanarayan Kaushik2, Yan Wen2, Maggie Fung2, and Darryl B Sneag1
1Radiology, Hospital for Special Surgery, New York, NY, United States, 2GE Healthcare, Chicago, IL, United States
1Radiology, Hospital for Special Surgery, New York, NY, United States, 2GE Healthcare, Chicago, IL, United States
Synopsis
Advances in deep-learning algorithms aiming to improve image quality have not yet been well studied for their use in clinical interpretation. In this study, we compared interobserver agreement and image quality for lumbar spine (L-spine) MRI assessment of 3D T2-weighted fast spin echo (T2w-FSE) MRI, with and without deep learning (DLRecon) reconstructions, as well as standard-of-care (SOC) 2D T2w-FSE MRI. This pilot study demonstrated that interobserver agreement for variables of interest was good to very good regardless of reconstruction or sequence type, and overall image quality of DLRecon was not inferior despite significant reduction in scanning time.
Introduction
Deep learning (DL)/machine learning applications in radiology have not only impacted disease detection or classification (1, 2), but have also positively impacted image quality via noise/artifact reduction and super-resolution reconstruction (3). While DL image enhancements in applications such as MRI of the knee (4) and coronary CT angiography (5) have been evaluated, their impact on routine L-spine MRI has remained largely unexplored. L-spine MRI typically includes multiple, high-resolution 2D T2w-FSE acquisitions (sagittal, coronal, and 2-3 oblique axials) with total scan times nominally reaching 25 minutes. While a single, isotropic, 3D T2w-FSE scan may potentially provide these multiplanar images at significantly shorter total acquisition time (about 6-8 minutes)(6), it is challenging to match the SNR and spatial resolution of 2D T2w-FSE using standard reconstruction techniques. This study’s objective was to evaluate the application of a DLRecon algorithm (7) to enhance 3D T2w-FSE of the L-spine. We hypothesized that while providing a beneficial decrease in acquisition time, DLRecon 3D T2w-FSE would also allow for similar interobserver agreement and overall improved image quality compared to both standard 3D reconstruction, and SOC 2D T2w-FSE.Methods
Under an IRB-approved study, MR images of 15 patients who underwent routine lumbar spine MRI, with both SOC 2D and isotropic 3D T2w-FSE sequences acquired, were retrospectively reviewed. Scans were acquired using a clinical 3T scanner (Signa Premier, GE Healthcare) with a 60-channel table array and a 30-channel anterior array (GE AIR Coil). Sequences evaluated included the following: 1) 3D T2w-FSE (0.8x0.8x0.8mm, TR/TE=1550/90ms, time=6.3-7.2 minutes); 2) 2D T2w-FSE axial (parallel to L4-5 disc space) (0.5x0.9x3.5mm, TR/TE=2500-5000/110ms); and 3) 2D T2w-FSE sagittal (same parameters as axial) (Fig 1). A 2D DLRecon algorithm (AIR Recon DL) (7), with denoising and sharpening properties, was retrained for 3D image reconstruction. 3D SOC, 3D DLRecon, and 2D SOC images were obtained, anonymized, and then randomized for evaluation by three readers (two musculoskeletal fellowship-trained attending radiologists and a musculoskeletal radiology fellow). Images were evaluated for presence of motion artifact, ringing/flow artifact, overall image quality, central stenosis, foraminal stenosis, disc degeneration, annular fissure, presence of facet synovial cysts and disc herniations using a predefined 3-6 point grading scale personalized to each variable of interest validated by current literature. Statistical Analysis: Inter-rater agreement for each variable of interest graded was evaluated using Conger’s kappa (K) as well as unadjusted percent agreement (SAS v9.4, Cary, NC).Results
Preliminary results showed that interobserver agreement for the major imaging variables of interest was overall similar between all 3 sequences with Conger’s kappa for foraminal stenosis ranging from 0.62-0.88 for DLRecon 3D T2w-FSE, 0.57-0.82 for SOC 3D T2w-FSE and 0.62-0.88 for SOC 2D FSE (Fig 2). Similarly, the Conger’s kappa for central stenosis at L4-5 was 0.85, 0.82 and 0.90 respectively, ranged from 0.54-0.84, 0.51-0.87, 0.27-0.84 respectively for disc herniation (L3-4 and L4-5) and ranged from 0.47-0.65, 0.55-0.82 and 0.59-0.84 respectively for disc degeneration (L3-4 to L5-S1). Evaluation of overall imaging quality characteristics showed that 3D DLRecon images were more often graded as of excellent quality (17/44) when compared to SOC 3D T2w-FSE (4/44), 2D axial (5/44) and 2D sagittal (1/44) sequences (Fig 3). Additionally, DLRecon 3D T2w-FSE cases were most often devoid of motion artifact (33/44) compared to SOC 3D T2w-FSE (20/44), 2D axial (16/44) and 2D sagittal (9/44) sequences (Fig 3). Anecdotally, 3D imaging in scoliosis patients made evaluation of foraminal and central stenosis facile, given the ability to create multiplanar reformatted images (Fig 4).Discussion
This preliminary, pilot study demonstrated that graded variables of interest showed overall moderate to very good interobserver agreement and this was comparable between each type of acquisition and reconstruction. Interestingly, despite facilitating marked reduction in sequence acquisition time, DLRecon 3D T2w-FSE demonstrated a similar interobserver agreement when compared to 2D imaging and was also often graded as having excellent image quality. While not directly evaluated, it can be theorized that speed and confidence of interpretation can be improved through superior image quality. This study was limited by its small sample size, but final evaluation will evaluate 35 cases per observer (based on a pre-study power analysis for all variables). Additionally, all studies were performed at the single magnet field strength of 3T, and the DLRecon algorithm was not applied to 2D images due to logistical unavailability at the time. Finally, these early results are not generalizable to the thoracic or cervical spine.Conclusion
3D T2w-FSE imaging of the lumbar spine, when enhanced by a deep learning algorithm, is promising to facilitate substantial time savings and the ability to reformat sequences freely without compromising interobserver agreement for evaluation of clinically relevant pathology. A similar acquisition and reconstruction method may demonstrate even greater benefits when applied to the cervical spine region where neural foramina are more obliquely oriented and where multiplanar reformations become more critical to ensure accuracy.Acknowledgements
No acknowledgement found.References
1. McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, et al. Deep Learning in Radiology. Acad Radiol. 2018;25(11):1472-80. doi: https://doi.org/10.1016/j.acra.2018.02.018. 2. Mazurowski MA, Buda M, Saha A, Bashir MR. Deep learning in radiology: An overview of the concepts and a survey of the state of the art with focus on MRI. Journal of magnetic resonance imaging : JMRI. 2019;49(4):939-54. Epub 2018/12/21. doi: 10.1002/jmri.26534. PubMed PMID: 30575178. 3. Higaki T, Nakamura Y, Tatsugami F, Nakaura T, Awai K. Improvement of image quality at CT and MRI using deep learning. Jpn J Radiol. 2019;37(1):73-80. doi: 10.1007/s11604-018-0796-2. 4. Chaudhari AS, Fang Z, Kogan F, Wood J, Stevens KJ, Gibbons EK, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med. 2018;80(5):2139-54. Epub 2018/03/26. doi: 10.1002/mrm.27178. PubMed PMID: 29582464. 5. Tatsugami F, Higaki T, Nakamura Y, Yu Z, Zhou J, Lu Y, et al. Deep learning–based image restoration algorithm for coronary CT angiography. Eur Radiol. 2019;29(10):5322-9. doi: 10.1007/s00330-019-06183-y. 6. Lee S, Jee WH, Jung JY, Lee SY, Ryu KS, Ha KY. MRI of the lumbar spine: comparison of 3D isotropic turbo spin-echo SPACE sequence versus conventional 2D sequences at 3.0 T. Acta Radiol. 2015;56(2):174-81. Epub 2014/02/21. doi: 10.1177/0284185114524196. PubMed PMID: 24553584. 7. Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline2020 August 01, 2020:[arXiv:2008.06559 p.]. Available from: https://ui.adsabs.harvard.edu/abs/2020arXiv200806559LFigures
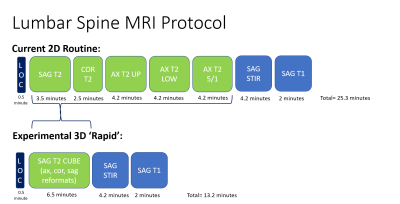
Fig 1. Top row illustrates our institution’s
current 3T lumbar spine MRI protocol, which involves 2-dimenisional (2D) T2
weighted acquisitions (including three separate axial batches, parallel to the
disc space, along the craniocaudal extent of the spine) as well as 2D sagittal
STIR and T1-weighted acquisitions. The experimental, 3D ‘rapid’ protocol
replaces the five separate 2D T2 acquisitions with a single sagittal
acquisition that can then be reformatted. Note the >10
minute time
savings.

Fig. 2 Top Row, sagittal T2-weighted images of
the lumbar spine categorized by sequence demonstrated markedly improved image
quality using the DLRecon
algorithm for 3D imaging:
A:
2D T2 standard of care (SOC) T2-weighted fast spin echo (T2w-FSE) B: 3D
standard of care (SOC) T2w-FSE C: Deep learning reconstructed (DLREcon) 3D
T2w-FSE
Bottom Row, axial T2-weighted images of
the lumbar spine, categorized by sequence, demonstrate superior image quality
with the DLRecon
algorithm for 3D imaging:
D: 2D T2 standard of care (SOC) E: 3D SOC
T2w-FSE F: DLRecon 3D
T2w-FSE
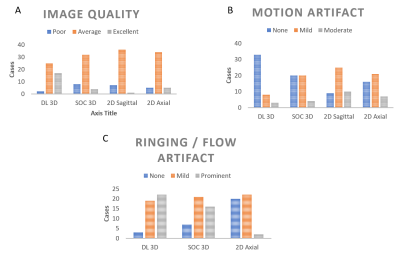
Fig. 3 Bar
charts demonstrate the distribution of variables of interest graded on a 3
point-scale categorized by sequence (DLRecon)
3D T2-weighted fast spin echo (T2w-FSE), standard of care (SOC) 3D T2w-FSE, 2D
sagittal and 2D axial sequences:
A.Image
quality grading (poor, average, excellent)
B.Motion
artifact grading (none, mild, moderate)
C.Ringing/flow
artifact grading (none, mild, prominent)
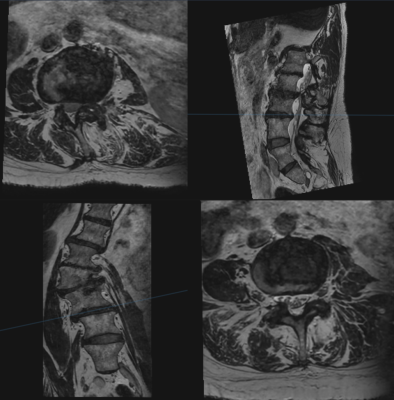
Fig. 4 Multiplanar reformations of 3D DLRecon T2
lumbar spine in a patient with moderate scoliosis demonstrating the ability to
optimally evaluate each level using orthogonal planes.