0802
MR-Guided Blood-brain Barrier Opening Induced by Rapid Short-pulse Ultrasound on Non-human Primates1Lauterbur Imaging Research Center, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2The Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, shenzhen, China, 3Research center for medical AI,Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 4Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province, Shenzhen, China
Synopsis
The BBB has been opened with millisecond ultrasound in kinds of animals for researching of neurological diseases therapy. Rapid short-pulse (RaSP) ultrasound with a microsecond sequence has been proposed as a minimally disruptive and efficient method for BBB opening in mice and rats. This work quantitatively evaluate the feasibility and safety of BBB opening in non-human primate with RaSP by contrast enhanced MRI. The relative signal enhancement in RaSP with 6% energy deposition reached more than 60% of that with 10 ms long pulse (LP), which shows that RaSP is a practical method for BBB opening in a large-animal model.
Purpose
Verifying the feasibility and safety of BBB opening on Non-human primate induced by RaSP ultrasound with a single element transducer based on MRI.Introduction
The blood-brain barrier (BBB) is a selectively permeable boundary separating the parenchyma of the central nervous system (CNS) from the systemic circulation1.Temporarily opening the BBB for drug delivery with low-intensity pulsed ultrasound combining with microbubbles(MBs) with Magnetic Resonance Imaging (MRI) guidance has shown promise in preclinical studies on animal models and even in human subjects2-5. Despite of these, the potential for side- effects, such as neuronal damage, red blood cell extravasation, and neuroinflammation6-7, albumin penetration into the brain should be assessed before this technique is applied clinically. Recently, Morse et al8 reported on a RaSP ultrasound for BBB opening on mice that can reduce the duration of the permeability change and albumin released into the brain, while the dextran delivery dosage was not significantly reduced. Following their research, this work evaluated the BBB opening quantitatively in both RaSP and LP trials by contrast enhanced MRI in non-human primate.Method
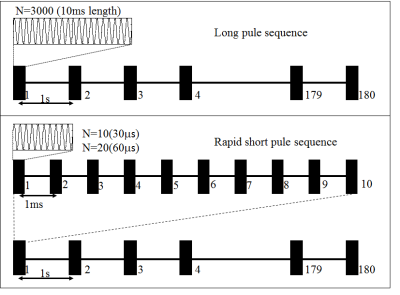
The experiment was under the approval of the Institutional Review Board. Two monkeys (M21 and M22) were employed in this study. The monkey was anesthetized and maintained with a ventilator, lie in a holder on the MRI bed with respiratory and blood oxygen monitoring and warmed with hydrothermal blanket, as shown in Fig. 1. The monkey was first scanned with T1-weighted FSE (TR/TE = 600/11 ms; flip angle= 90, echo length:3),T2*-weighted GRE (TR/TE =500/20 ms; flip angle= 30) and T2-weighted FSE(TR/TE = 5000/75 ms; flip angle= 90,echo length:16 ) for baseline with spatial resolution: 0.4×0.4×2mm. The sonication was then carried out by a single element spherical transducer (diameter: 50mm; focus length: 55mm) of 300kHz with a bolus of microbubble (Sonovue, with 0.15ml/kg) intravenously injection from leg at the beginning of sonication. The peak negative power of sonication was about 0.56Mpa (calibrated in water), the ultrasound protocols (RaSP30, RaSP60, LP10) were showed in Fig.2. After sonication, contrast enhanced agent Gd-DTPA was injected, and the temporal course of signal enhancement was got by T1-weighted FSE. At the end, T2*-weighted GRE and T2-weighted FSE sequences were used for edema and hemorrhage detection9. Two ROIs (at the focused hemisphere and the opposite hemisphere) were drawn in the T1w images. The signal intensity was first normalized to water to avoid signal fluctuation. Signal enhancement maps at each time point were defined by the relative signal change against the image acquired before Gd-DTPA was injected:$$$\triangle{S}=\frac{S(t)-S(0)}{S(0)}$$$
Student’s t-test was performed on the relative signal enhancement between two ROIs at different time points in each trial to see whether the contrast enhancement appeared in the sonicated area to check that the BBB was opened in each trail.
Results
The feasibility of opening the BBB by RaSP was demonstrated by contrast enhancement around the targeted focal area after ultrasound sonication in all experiments, as shown in Fig.3. The relative signal enhancement in ROIF after BBB opening was significantly higher than that in ROIC for all the experiment groups (p<0.01). In all the study groups, the differences between relative signal enhancement in ROIF and ROIC were positively correlated with the burst length of pulses in each sonication protocol. The relative signal enhancement of the same protocol showed good consistency between different experiment groups -in each animal model, as illustrated in Fig.4. The relative signal enhancement in RaSP30 and RaSP60 reached more than 30% or 60% of that with LP10 sonication, while the energy deposition in RaSP30 and RaSP60 was only 3% and 6% of LP10. There was no observable change in the T2w/T2*w MRI images for RaSP30 and RaSP60.Conclusion and Discussion
This work demonstrated the feasibility and safety of rapid short-pulse ultrasound in opening the BBB blood-brain barrier in a non-human primate model, which shows RaSP maybe preferable to the traditional long pulses. Studies are needed with varying acoustic power and burst length and MBs parameters including size and dosage to find the optimized parameters for shorter BBB recovery time.Acknowledgements
This work was supported by the Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province.References
1. Meng Y, Pople CB, Lea-Banks H, et al. Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. Journal of controlled release : official journal of the Controlled Release Society 2019;309:25-36
2. Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220(3):640-646.
3. McDannold N, Livingstone M, Top CB, Sutton J, Todd N, Vykhodtseva N. Preclinical evaluation of a low-frequency transcranial MRI-guided focused ultrasound system in a primate model. Phys Med Biol 2016;61(21):7664-7687.
4. Downs ME, Buch A, Sierra C, et al. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task (vol 10, e0125911, 2015). PloS one 2015;10(6).
5. Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun 2018;9.
6. Todd N, Angolano C, Ferran C, Devor A, Borsook D, McDannold N. Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood-brain barrier. Journal of Controlled Release. 2020;324:450-9.
7. Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(1):E75-E84.
8. Morse SV, Pouliopoulos AN, Chan TG, Copping MJ, Lin JL, Long NJ, et al. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology. 2019;291(2):458-65.
9. Morocz IA, Hynynen K, Gudbjartsson H, Peled S, Colucci V, Jolesz FA. Brain edema development after MRI-guided focused ultrasound treatment. J Magn Reson Imaging. 1998;8(1):136-42
Figures