0788
ChEST: A novel model measuring both Chemical Exchange and Susceptibility Tensor from resonance frequency shift1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Department of Radiology and Radiological Science, Division of MR Research, Johns Hopkins Medicine, Baltimore, MD, United States
Synopsis
We introduce ChEST, a novel model that can estimate both chemical exchange and magnetic susceptibility tensor effects from resonance frequency shift. For reconstruction, an iterative algorithm is designed to solve the inverse problem of the new model. When tested using numerical simulation datasets, our method successfully generated mean magnetic susceptibility, magnetic susceptibility anisotropy, principal eigenvector, and chemical exchange maps in high accuracy as compared to conventional methods. Application to in-vivo human brain was conducted, revealing promising outcomes.
Introduction
Quantitative susceptibility mapping (QSM) algorithms assume that the resonance frequency shift is attributed only to the isotropic magnetic susceptibility.1 However, additional sources such as susceptibility anisotropy2 and chemical exchange (CE)3 have been demonstrated to exist. Susceptibility tensor imaging (STI) extended the QSM model to include susceptibility anisotropy, revealing fiber orientation information.4 Recently, a multi-directional approach for separating susceptibility and chemical exchange was developed, suggesting the potentials of developing a more complete model of frequency shift.5 In this study, a novel model that encompasses both Chemical Exchange and Susceptibility Tensor (ChEST) is proposed and an iterative algorithm that solves the inverse problem of the model is developed. Numerical simulation and in-vivo results are included.Methods
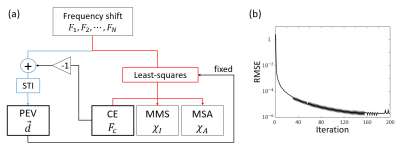
[ChEST model] The frequency shifts with N different B0 directions in the subject frame of reference can be modeled as the sum of frequency shifts from chemical exchange and susceptibility tensor:4, 5$$\triangledown{F_i}={FT}^{-1}\left\{\frac{1}{3}\overrightarrow{H}_i^TFT\left(\chi\right)\overrightarrow{H}_i-\overrightarrow{k}\cdot\overrightarrow{H}_i^T\frac{\overrightarrow{k}^TFT\left(\chi\right)\overrightarrow{H}_i}{\left|\overrightarrow{k}\right|^2}+FT\left(F_c\right)\right\},{\qquad}[Eq. 1]$$ where $$$FT$$$ and $$$FT^{-1}$$$ mean the Fourier transform and its inverse, $$$\overrightarrow{H}_i=\begin{bmatrix}H_{1i}&H_{2i}&H_{3i}\end{bmatrix}^T$$$ is the magnetic strength vector along each B0 (i = 1,2,…, and N), $$$\triangledown{F_i}$$$ is the total frequency shift for the B0 direction, $$$F_c$$$ is CE, and $$$\chi$$$ is the susceptibility tensor. CE is assumed to be orientation independent. This equation can be reformated as follows: $$\triangledown{F_i}={FT}^{-1}\begin{bmatrix}\frac{1}{3}H_{1i}^2-\overrightarrow{k}\cdot\overrightarrow{H}_i\frac{k_1H_{1i}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{1i}H_{2i}-\overrightarrow{k}\cdot\overrightarrow{H}_i\frac{k_1H_{2i}+k_2H_{1i}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{3i}^2-\overrightarrow{k}\cdot\overrightarrow{H}_i\frac{k_3H_{3i}}{\left|\overrightarrow{k}\right|^2}\end{bmatrix}{FT}\begin{bmatrix}\chi_{11}\\\chi_{12}\\\chi_{13}\\\chi_{22}\\\chi_{23}\\\chi_{33}\\F_c\end{bmatrix}.{\qquad}[Eq. 2]$$ When multiple B0 directional data are acquired, Equation 2 can be rewritten as follows:6 $$\begin{bmatrix}\triangledown{F_1}\\\triangledown{F_2}\\{\vdots}\\\triangledown{F_N}\end{bmatrix}={FT}^{-1}\begin{bmatrix}\frac{1}{3}H_{11}^2-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_1H_{11}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{11}H_{21}-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_1H_{21}+k_2H_{11}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{31}^2-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_3H_{31}}{\left|\overrightarrow{k}\right|^2}\\\frac{1}{3}H_{12}^2-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_1H_{12}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{12}H_{22}-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_1H_{22}+k_2H_{12}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{32}^2-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_2H_{32}}{\left|\overrightarrow{k}\right|^2}\\&&\vdots\\\frac{1}{3}H_{1N}^2-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_1H_{1N}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{1N}H_{2N}-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_1H_{2N}+k_2H_{1N}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{3N}^2-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_3H_{3N}}{\left|\overrightarrow{k}\right|^2}\end{bmatrix}{FT}\begin{bmatrix}\chi_{11}\\\chi_{12}\\\chi_{13}\\\chi_{22}\\\chi_{23}\\\chi_{33}\\F_c\end{bmatrix}.{\qquad}[Eq. 3]$$ However, even with a large number of B0 directions, the maximum rank of Equation 3 is still 6 because all components of the matrix can be represented as a linear combination of $$$H_{1i}^2$$$, $$$H_{2i}^2$$$, $$$H_{3i}^2$$$, $$$H_{1i}H_{2i}$$$, $$$H_{1i}H_{3i}$$$, $$$H_{2i}H_{3i}$$$ (i.e., $$$H_{1i}^2+H_{2i}^2+H_{3i}^2=1$$$). To overcome this rank deficiency, we posed an assumption that the anisotropy is cylindrically symmetric.7 Then, the susceptibility tensor ($$$\chi$$$) can be described as:$$\chi=(\chi_I-\frac{\chi_A}{3})I+\chi_A\overrightarrow{d}\overrightarrow{d}^T,{\qquad}[Eq. 4]$$ where $$$\chi_I$$$ is the mean magnetic susceptibility (MMS), $$$\chi_A$$$ is the magnetic susceptibility anisotropy (MSA), $$$I$$$ is an identity matrix, and $$$\overrightarrow{d}=\begin{bmatrix}d_1&d_2&d_3\end{bmatrix}^T$$$ is the principal eigenvector (PEV). Then, Equation 3 can be expressed as: $$\begin{bmatrix}\triangledown{F_1}\\\triangledown{F_2}\\{\vdots}\\\triangledown{F_N}\end{bmatrix}=A\begin{bmatrix}1\\0\\0\\1\\0\\1\end{bmatrix}\chi_I+A\begin{bmatrix}d_1^2-\frac{1}{3}\\{d_1d_2}\\{d_1d_3}\\d_2^2-\frac{1}{3}\\{d_2d_3}\\d_3^2-\frac{1}{3}\end{bmatrix}\chi_A+\begin{bmatrix}1\\1\\{\vdots}\\1\end{bmatrix}F_c,{\qquad}[Eq. 5]$$ where $$$A={FT}^{-1}\begin{bmatrix}\frac{1}{3}H_{11}^2-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_1H_{11}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{11}H_{21}-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_1H_{21}+k_2H_{11}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{31}^2-\overrightarrow{k}\cdot\overrightarrow{H}_1\frac{k_3H_{31}}{\left|\overrightarrow{k}\right|^2}\\\frac{1}{3}H_{12}^2-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_1H_{12}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{12}H_{22}-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_1H_{22}+k_2H_{12}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{32}^2-\overrightarrow{k}\cdot\overrightarrow{H}_2\frac{k_2H_{32}}{\left|\overrightarrow{k}\right|^2}\\&&\vdots\\\frac{1}{3}H_{1N}^2-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_1H_{1N}}{\left|\overrightarrow{k}\right|^2}&\frac{2}{3}H_{1N}H_{2N}-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_1H_{2N}+k_2H_{1N}}{\left|\overrightarrow{k}\right|^2}&\cdots&\frac{1}{3}H_{3N}^2-\overrightarrow{k}\cdot\overrightarrow{H}_N\frac{k_3H_{3N}}{\left|\overrightarrow{k}\right|^2}\end{bmatrix}FT$$$. To resolve the multiplication between $$$\overrightarrow{d}$$$ and $$$\chi_A$$$ in the equation, an iterative method is developed to estimate $$$\chi_I$$$, $$$\chi_A$$$, $$$\overrightarrow{d}$$$, and $$$F_c$$$ . The iteration consists of two steps. In the first step, STI is performed with fixed $$$F_c$$$, and then the principal eigenvector $$$\overrightarrow{d}$$$ is calculated by eigenvalue decomposition. In the second step, with $$$\overrightarrow{d}$$$ fixed, $$$\chi_I$$$, $$$\chi_A$$$, and $$$F_c$$$ are fitted with Equation 5 using the least-squares method. These two steps are iterated until the difference between measured frequency shift and predicted frequency shift are less than a threshold, producing MMS, MSA, PEV, and CE (Fig. 1a).[Simulation] A brain-shaped phantom is designed with the cylindrically symmetric susceptibility tensor and chemical exchange. The chemical exchange is assumed to have a lower contrast range than that of MMS referenced from a recent study5 (e.g. iron oxide: CE = -39 ppb and susceptibility = 300 ppb). Then, twelve directional datasets are generated ( $$$\theta$$$ = [0°, 17.2°, 23.5°, 15.5°, 24.2°, 7.9°, 12.9°, 15.1°, 13.9°, 20.7°, 20.8°, 25.4°] and $$$\phi$$$ = [0°, -14°, -32.5°, -155°, -153.2°, 85.1°, 12.9°, -179.7°, -96.4°, -88.4°, -76.2°, -111.4°]). The ChEST is applied to reconstruct the MMS, MSA, PEV, and CE maps. Simulation is conducted with and without Gaussian noise (SNR = 100). A conjugate gradient algorithm (tolerance: 10-3) is used for least-squares estimation with regularization to constrain MSA in white matter similar to MMSR-STI8 ( $$$\alpha=10$$$). It takes 200 iterations to converge (Fig. 1b). For comparison, MMSR-STI8 and STI4 reconstructions are also performed.
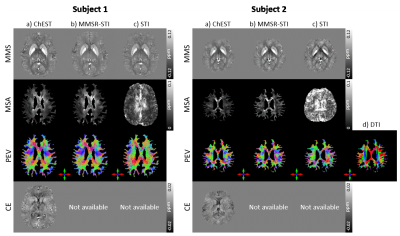
[In-vivo application] To demonstrate the feasiblity of applying ChEST to in-vivo data, two existing datasets are utilized (Subject 1: 12 directional QSM challenge 2016 data;9 Subject 2: 12 directions, 3D GRE, FOV = 216×216×216 mm3, resolution = 1.5×1.5×1.5 mm3, TR/TE = 40/25 ms, FA = 15° , TA = 11:03 per acquisition.8)
Results
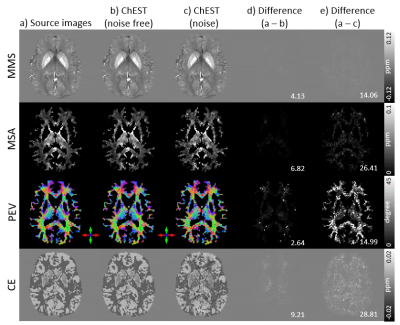
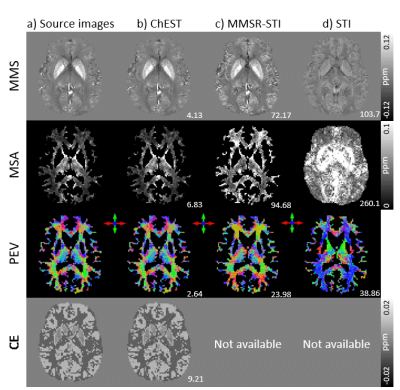
As demonstrated in Figure 2, the proposed ChEST method successfully reconstructs MMS, MSA, PEV, and CE maps in the noise-free condition. Even with additive Gaussian noise, ChEST produces all maps with small errors. Figure 3 illustrates the comparison results for the ChEST and conventional algorithms. When compared to the conventional STI algorithms that exclude the chemical exchange in their models, the proposed method generates the maps with lower NRMSE values. In Figure 4, the in-vivo results are displayed. The CE map shows a much smaller contrast range compared to those of MMS and MSA, confirming our previous assumption in QSM that the contribution of CE is much smaller than that of susceptibility. The other susceptibility-related maps are consistent with MMSR-STI.Conclusion and Discussion
In this study, we introduced an integrated model for frequency shift, proposing ChEST, a novel method that estimates both chemical exchange and susceptibility tensor. Compared to the previous approaches, our method successfully reconstructs MMS, MSA, PEV, and CE maps. In the in-vivo results, the CE map showed a smaller contrast range than the MMS and MSA maps, agreeing with our recent measurements.5Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT) (No. NRF-2018R1A2B3008445, NRF-2017M3C7A1047864).References
1. De Rochefort, L., Brown, R., Prince, M. R., & Wang, Y. I. (2008). Quantitative MR susceptibility mapping using piece‐wise constant regularized inversion of the magnetic field. Magnetic Resonance in Medicine, 60(4), 1003-1009.
2. Lee, J., Shmueli, K., Fukunaga, M., van Gelderen, P., Merkle, H., Silva, A. C., & Duyn, J. H. (2010). Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proceedings of the National Academy of Sciences, 107(11), 5130-5135.
3. Shmueli, K., Dodd, S. J., Li, T. Q., & Duyn, J. H. (2011). The contribution of chemical exchange to MRI frequency shifts in brain tissue. Magnetic Resonance in Medicine, 65(1), 35-43.
4. Liu, C. (2010). Susceptibility tensor imaging. Magnetic Resonance in Medicine, 63(6), 1471-1477.
5. Eun, H., Jeong, H., Lee, J., Shin, H. G., & Lee, J. (2020). A geometric approach to separate the effects of magnetic susceptibility and chemical shift/exchange in a phantom with isotropic magnetic susceptibility. Magnetic Resonance in Medicine, 85(1), 281-289.
6. Liu, T., Spincemaille, P., De Rochefort, L., Kressler, B., & Wang, Y. (2009). Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magnetic Resonance in Medicine, 61(1), 196-204.
7. Wharton, S., & Bowtell, R. (2012). Fiber orientation-dependent white matter contrast in gradient echo MRI. Proceedings of the National Academy of Sciences, 109(45), 18559-18564.
8. Li, X., & van Zijl, P. C. (2014). Mean magnetic susceptibility regularized susceptibility tensor imaging (MMSR‐STI) for estimating orientations of white matter fibers in human brain. Magnetic resonance in medicine, 72(3), 610-619.
9. Langkammer, C., Schweser, F., Shmueli, K., Kames, C., Li, X., Guo, L., ... & Buch, S. (2018). Quantitative susceptibility mapping: report from the 2016 reconstruction challenge. Magnetic resonance in medicine, 79(3), 1661-1673
Figures