0777
Calibration-free pTx of the human heart at 7T via 3D universal pulses1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 3Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
We demonstrate calibration free universal 4kT-points pulse design to achieve a subject independent, homogeneous flip-angle within the human heart at 7T. The proposed universal pulse was computed offline based on 22 three-dimensional B1+-maps of 20 volunteers with varying BMI and age (two of them were scanned twice with different coil placement). The optimized universal pulse was successfully applied experimentally to one volunteer from the library and four new unseen volunteers. In total we have analyzed 27 B1+ maps from 24 volunteers. Experimental data at 7T validate the B1+ predictions and demonstrate successful plug-and-play 3D pTx of the human heart.
Purpose
Ultra-high field MR is often limited by inhomogeneities of the transmit field (B1+), which can be compensated by parallel transmission (pTx)1. In contrast to pTx methods that require subject-specific (SubjSpec) B1+-maps, pre-computed calibration-free universal pulses (UPs) were proposed in the human head2 to save precious scan time. B1+ mapping and pTx of the human heart is generally more challenging and time consuming because of multiple manifestations of physiological motion3,4. However, a one-fits-all-solution for the human heart has not been shown so far and due to large inter-subject variations, it was not clear if UPs work in the human body at all. Here, we demonstrate for the first time the feasibility of using UPs in the human body to achieve homogeneous flip-angle (FA) distributions throughout the heart. The UP was computed offline using 22 B1+-maps of 20 healthy volunteers and was successfully validated experimentally at 7T in one volunteer from the library and in four unseen volunteers.Methods
MRI was performed on a Siemens Magnetom 7T scanner according to an approved IRB protocol in 24 healthy volunteers (14M/10F, mean:31y, range:21-66y) with varying BMI (mean:24kg/m2, range:20-29kg/m2). The scans were performed with a commercial, certified 32-element MRI.TOOLS body coil array driven in 8TX/32RX mode. Local/global SAR limits in first level mode (IEC60601-2-33) were complied by limiting the radio-frequency (RF) power of each transmit channel. Relative 3D B1+-maps of the thorax were acquired3 with a radial phase-encoding trajectory5 under free breathing (nominal FA=20°, TE/TR=2.02/40ms, FOV=250x312x312mm3, resolution=(4mm)3, 256 RPE-lines, TA=205s). Shallow breathing allowed reconstruction of non-respiration resolved B1+-maps without motion-induced artifacts3. SubjSpec magnitude/phase (MP) and 4kT-point pulses were designed using the small-tip-angle approximation in MATLAB for the 3D heart volume4,6. This approach was extended for multiple B1+ maps, inspired by the design of UPs in the human head2, to achieve a homogeneous FA in each heart$$$\:ROI\:$$$for each kT-point solving$$\min\limits_{b}\frac{1}{2}\left\|\sum_{m=1}^{N_m}\left(|m_d|-|\sum_{ch=1}^{N_{ch}}B_{1,ch,m}^+A(K)b_{ch}|\right)\right\|^2_{ROI}+\frac{\beta}{2}\left\|b\right\|^2$$with$$$\:b$$$:RF-weights,$$$\:A(K)$$$:excitation-matrix at k-space location$$$\:K$$$,$$$\:N_m$$$:number of B1+ maps,$$$\:N_{ch}$$$:number of transmit channels,$$$\:B_{1,ch,m}^+$$$:B1+ map,$$$\:m_d$$$:desired FA,$$$\:\beta$$$:regularization term. The 4kT-UP (duration=0.96ms) was computed offline in 10min using 22 B1+-maps from 20 volunteers (2 volunteers were scanned twice with different coil placement). The 4kT-UP’s performance was analyzed using the root-mean-squared-error, the RF power and the coefficient of variation (CV)1 in the heart ROIs. In addition, a leave-one-subject-out cross-validation was performed to analyze the impact of each B1+-map on the UP. High-resolution 3D gradient-echo scans (nominal FA=10°, TE/TR=1.75/3.7ms, FOV=250x312x312mm3, resolution=(1.4mm)3, 256 RPE-lines, TA=333s) have been acquired to validate the SubjSpec-4kT pulses in all 24 volunteers. The precomputed 4kT-UP was applied in four unseen volunteers (2F/2M) and in one re-scanned volunteer from the library with different coil placement.Results and Discussion
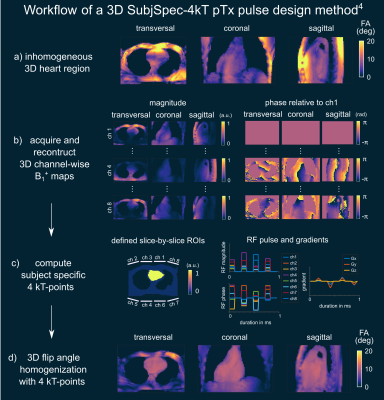
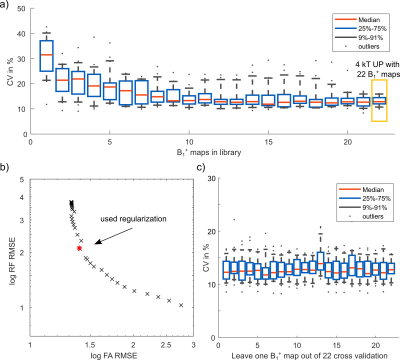
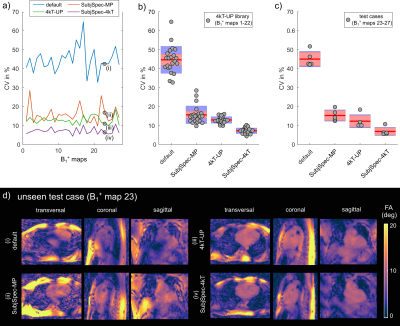
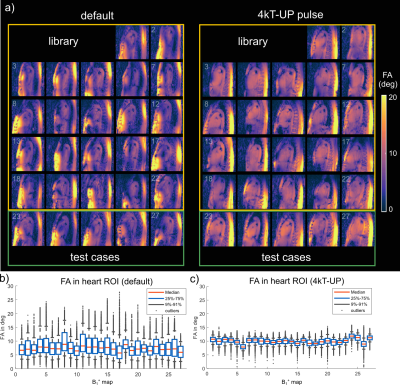
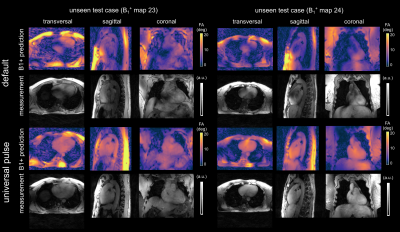
Fig.1a-d illustrates the workflow of a 3D SubjSpec-4kT design4 starting from a default B1+ phase shim (equal magnitude). The starting phase yields variable FA homogeneity across the volunteers (CV=64-34%) with strong dropouts in some volunteers that can be reduced with SubjSpec-4kT pulses (CV=6-11%) but requires SubjSpec B1+-maps, which motivates the use of UP. Fig.2a shows the performance of 4kT-UPs designed for an increasing number of B1+ maps in the library (1,1-2,1-3,…,1-22). Note that all UPs were evaluated for all 22 B1+ maps. As expected, adding more B1+-maps to the library decreases the mean CV from 31.5% (1 B1+ map) to 12.8%, range:10.7-15.8% (22 B1+ maps). Fig.2b-c show the tuning of the regularization parameter and a robust leave-one-subject-out cross-validation of the proposed 4kT-UP marked in Fig.2a. Fig.3a-c show the resulting CV in the heart of 27 B1+ maps (22 from the library and 5 test-cases) using four excitation settings: default, SubjSpec-MP, SubjSpec-4kT and 4kT-UP. On average, the 4kT-UP performs better than SubjSpec-MP (12.8% vs. 18%) and does not result in severe signal drops in the heart, typically indicated by elevated CV values >25%. As expected, further improvement in CV was achieved by using SubjSpec-4kT pulses (7%). The same trend was observed for the test-cases (Fig.3c). Fig.3d shows one representative unseen test-case. The 4kT-UP resulted in good FA homogeneity in the heart and, unexpectedly but consistently observed across all volunteers, also in surrounding tissues (e.g. aorta). Fig.4a qualitatively shows the B1+ prediction for a sagittal slice of all B1+ maps using the default shim and the proposed 4kT-UP. Note the large variation in anatomy and coil placement between B1+ prediction and the superb FA homogeneity. Quantitative FA distributions within the 3D heart ROIs are depicted in a boxplot showing the 4-fold reduction of FA spread in all B1+ prediction. Fig.5 shows the acquired, respiratory corrected 3D GRE images (acquired without cardiac gating) of two unseen test-cases using the proposed 4kT-UP. A close match between B1+ predictions and the 3D GRE images was observed, demonstrating the feasibility of calibration free pTx in the human heart for shallow breathing. Breathing patterns with larger respiratory amplitude will be focus of future work.Conclusion
This study demonstrates in vivo that 4kT-UP are highly suitable for calibration-free 3D heart FA homogenization at 7T despite large inter-subject variations due to varying age, BMI and coil placement. The proposed use of UPs allows plug-and-play pTx in the human heart without the need for lengthy and artefact prone calibration process and has the potential to push the limits of body imaging at 7T and above.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1 and GRK2260, BIOQIC.References
1) Padormo, F., Beqiri, A., Hajnal, J. V., and Malik, S. J. (2016) Parallel transmission for ultrahigh‐field imaging. NMR Biomed., 29: 1145– 1161. doi: 10.1002/nbm.3313
2) Gras, V., Vignaud, A., Amadon, A., et al. (2017) Universal pulses: A new concept for calibration‐free parallel transmission. Magn. Reson. Med., 77: 635-643. doi:10.1002/mrm.26148
3) Dietrich, S., Aigner, C.S., Kolbitsch, C., et al. (2020) 3D Free-breathing Multi-channel absolute B1+ Mapping in the Human Body at 7T, Magn. Reson. Med., doi:10.1002/mrm.28602
4) Aigner, C.S., Dietrich, S. and Schmitter, S. (2020) Three-dimensional static and dynamic parallel transmission of the human heart at 7T, NMR in Biomed., doi:10.1002/nbm.4450
5) Prieto, C., Uribe, S., Razavi, R., et al. (2010) 3D undersampled golden‐radial phase encoding for DCE‐MRA using inherently regularized iterative SENSE. Magn. Reson. Med., 64, pp. 514-526, doi:10.1002/mrm.22446
6) Cao, Z., Yan, X. and Grissom, W.A. (2016) Array‐compressed parallel transmit pulse design. Magn. Reson. Med., 76, pp. 1158-1169., doi:10.1002/mrm.26020
Figures