0773
Impact of inversion time for FLAIR acquisition on the T2-FLAIR mismatch detectability for IDH-mutant, non-CODEL astrocytomas1Neurosurgery, Osaka University Graduate School of Medicine, Suita, Japan, 2Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, Tokyo, Japan, 3Neurosurgery, Osaka City University Graduate School of Medicine, Osaka, Japan, 4Neurological Surgery, Wakayama Medical University, Wakayama, Japan, 5Neurosurgery, Osaka City General Hospital, Osaka, Japan, 6Diagnostic Radiology, Osaka International Cancer Institute, Osaka, Japan, 7Radiology, Osaka University Graduate School of Medicine, Suita, Japan, 8Division of Brain Tumor Translational Research, National Cancer Center Research Institute, Tokyo, Japan, 9Biomedical Research and Innovation, National Hospital Organization Osaka National Hospital, Osaka, Japan
Synopsis
Although the T2-FLAIR mismatch sign is a promising imaging marker specific for IDHmt, non-CODEL astrocytomas, its low sensitivity and NPV hinder its full clinical application. This study discovered that FLAIR acquisition with TI shorter than 2400 ms in 3T could overcome this issue and that the sensitivity and NPV improved to 67% and 74%. Tuning TI for FLAIR acquisition is such a simple technique that clinicians can easily incorporate this procedure into the daily workflow of glioma imaging. Our proposed method would provide a novel yet clinically feasible glioma imaging method in the era of personalized-molecular medicine of cancer.
INTRODUCTION
The T2-fluid-attenuated inversion recovery (FLAIR) mismatch sign has become one of the most prominent imaging features for IDH-mutant, 1p19q-non-codeleted (IDHmt, non-CODEL) astrocytomas. This sign is defined as a “complete/near-complete hyperintense signal on T2-weighted image (T2WI) and relatively hypointense signal on FLAIR except for hyperintense peripheral rim”. The low sensitivity of the T2-FLAIR mismatch sign, however, has been problematic. The authors have recently reported that long T1 and T2 effects of IDHmt, non-CODEL astrocytomas were the possible cause of the T2-FLAIR mismatch sign and that inversion time (TI) for acquiring FLAIR could have an impact on the appearance of the T2-FLAIR mismatch (1). That research revealed that optimum TI seems to lie between 2100 and 2700 ms under 3T. The current study aimed to test the hypothesis that TI shorter than 2400 ms under 3T for FLAIR can improve sensitivity without sacrificing specificity to detect the T2-FLAIR mismatch sign for identifying IDHmt, non-CODEL astrocytomas.METHODS
-
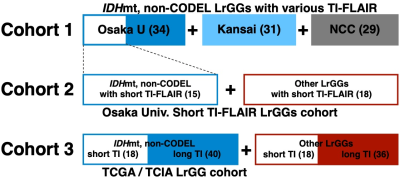
Patient
cohort (Figure 1) Cohort 1: 94 MRI studies from 76 histologically and molecularly
confirmed IDHmt, non-CODEL LrGGs from 6 institutions.
Cohort 2:
33 MRI studies from 31 histologically confirmed and molecularly characterized
LrGG from Osaka University Hospital. We collected the second cohort under the
restriction of FLAIR being acquired with TI < 2400 ms measured under 3T
Cohort 3:
112 MRI studies from 112 patients from the TCIA/TCGA dataset for LrGG.
- Genetic analysis Sanger sequencing or pyrosequencing assessed hotspot mutations of IDH1/2 (codon 132 of IDH1 and codon 172 of IDH2) and the TERT promoter (C228 and C250). 1p/19q co-deletion was detected either by copy number multiplex ligation-dependent probe amplification in a unified workflow or by fluorescence in situ hybridization (FISH). We obtained IDH and TERT promoter mutation and 1p19q co-deletion statuses for the TCIA/TCGA dataset from the report by Ceccarelli et al. (2).
- Detection of T2-FLAIR mismatch sign The presence or absence of the “T2-FLAIR mismatch sign” was evaluated in all cases by two independent readers. A consensus meeting between the two readers finally determined the presence or absence of the T2-FLAIR mismatch sign.
- Statistical analysis We performed statistical analysis using by Fisher’s exact test evaluated the statistical significance of contingency tables.
RESULTS
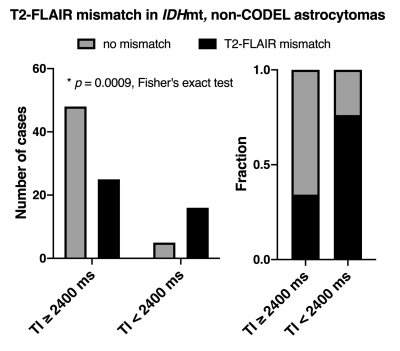
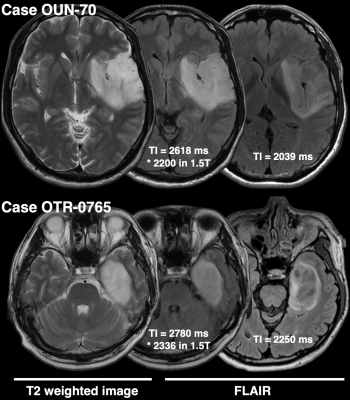
- TI of shorter than 2400 ms for FLAIR improves the detectability of T2-FLAIR mismatch sign in IDHmt, non-CODEL astrocytomas (Study from cohort 1) When FLAIR was acquired with TI shorter than 2400 ms in 3T, the fraction of the T2-FLAIR mismatch sign positive astrocytomas increased to 0.76 compared to 0.34 in cases where TI longer than 2400 ms was used (Figure 2, p = 0.0009, Fisher’s exact test). Some patients received more than two scans with FLAIR acquired using different TI. As shown in Figure 3, the ease of appreciating the T2-FLAIR mismatch sign was different between FLAIR with TI of shorter and longer than 2400 ms.
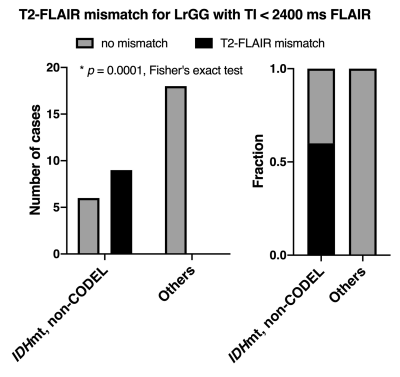
- Presence of the T2-FLAIR mismatch sign with FLAIR of TI < 2400 ms is specific for IDHmt, non-CODEL astrocytomas (Study from cohort 2) The specificity of the T2-FLAIR mismatch sign with FLAIR of TI < 2400 ms was 100% along with a 60% sensitivity for identifying IDHmt, non-CODEL astrocytomas among histologically confirmed LrGG (Figure 4).
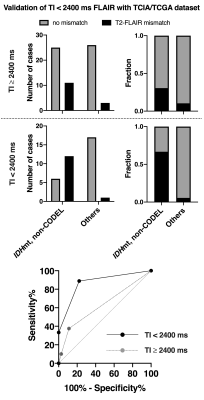
- Validation of the T2-FLAIR mismatch sign with FLAIR of TI < 2400 ms in the TCIA/TCGA dataset (Study from cohort 3) For the scans conducted in the TCIA/TCGA dataset with FLAIR using TI ≥ 2400 ms, the sensitivity, specificity, PPV, and NPV of the T2-FLAIR mismatch sign to identify IDHmt, non-CODEL astrocytomas were 30%, 92%, 80%, and 54%, respectively. For the scans conducted with FLAIR using TI < 2400 ms, those metrics improved to 67%, 94%, 92%, and 74%, respectively (Figure 5).
DISCUSSION
The T2-FLAIR mismatch sign is an image-based molecular diagnostic marker that clinicians can readily incorporate into clinical practice, as both T2WI and FLAIR are routine MRI protocols for gliomas. Although a mechanistic explanation of the T2-FLAIR mismatch sign is challenging, our recent study revealed that IDHmt, non-CODEL astrocytomas often contain tumors that exhibit long T1 and T2 effects, which phenomenon could be the leading cause of the T2-FLAIR mismatch sign. We set a threshold of TI as 2400 ms in 3T for acquiring FLAIR and studied the impact of different TIs on the diagnostic accuracy of the T2-FLAIR mismatch sign.As expected, FLAIR acquired with lower TI exhibited higher diagnostic accuracy both in our local dataset and in the TCIA/TCGA dataset used for validation purposes (Figures 3 and 4). Figure 2 illustrates the impact of TI on the presence or absence of the T2-FLAIR mismatch sign in the real clinical setting.
Our research highlights the importance of tailoring the FLAIR sequence in glioma imaging by lowering TI rather than merely using the conventional FLAIR sequence developed for stroke and neuroinflammation imaging.
CONCLUSION
We provide evidence that TI for FLAIR acquisition significantly impacts the diagnostic accuracy of the T2-FLAIR mismatch sign, especially sensitivity and NPV. Although external cohorts should validate our findings, we propose that it is necessary for LrGG imaging to acquire FLAIR with TI shorter than 2400 ms in 3T.Acknowledgements
This research was funded by the Japan Society for the Promotion of Science (19K09526), Takeda Science Foundation, and MSD Life Science Foundation. All scientific grants were for Manabu Kinoshita.References
1) Kinoshita M, Uchikoshi M, Sakai M, Kanemura Y, Kishima H, Nakanishi K. T2-FLAIR Mismatch Sign Is Caused by Long T1 and T2 of IDH-mutant, 1p19q Non-codeleted Astrocytoma. Magn Reson Med Sci (2020)bc.2019-0196. doi:10.2463/mrms.bc.2019-0196
2)
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS,
Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, et al.
Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of
Progression in Diffuse Glioma. Cell (2016) 164:550-563.
Figures