0771
Quantification of pyruvate recycling in brain tumor patients1Peak Center, Neurosurgery, Houston Methodist Hospital, Houston, TX, United States, 2UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Pyruvate recycling is the metabolic pathway that generates pyruvate, lactate and alanine from the tricarboxylic acid (TCA) cycle intermediates, oxaloacetate (OAA) and malate. It is active in the liver and the kidney. Although existence and origin of pyruvate recycling mechanism in human brain has been shown to be active, it still remains controversial. Here, we demonstrate that pyruvate recycling mechanism is active in human GBM patients by 13C isotopomer analysis of resected tumor tissues. We have developed a simple method to determine the relative flux of pyruvate recycling with respect to glycolysis using C2 lactate 13C isotopomers.
Introduction
Cancer cells are in the constant need of generating carbon skeletons for the synthesis of macromolecules for the growth and proliferation. Most of such carbon precursors such as malate and oxaloacetate are produced within the TCA cycle. It is necessary to have mechanisms that replenish these TCA cycle intermediates when they are withdrawn from the cycle for macromolecular synthesis. Pyruvate recycling pathway allows the generation of pyruvate from malate and OAA which can reenter the TCA cycle either via OAA or acetyl-CoA. Although occurrence of pyruvate recycling mechanism in human brain has been shown, its origin still remains controversial [1-3]. There has been no detailed prior study on quantification of pyruvate recycling in brain tumor patients. Here, we report the quantitative detection of pyruvate recycling pathway in malignant brain tumor patients through use of C2 lactate 13C NMR multiplets from the tumor tissues of tumor patients who were intravenously infused with [U-13C]glucose during the surgical resection of the tumor.Methods
Ten brain tumor patients (GBM=8, brain metastases=2) were enrolled for this study through the protocol approved by the University of Texas Southwestern Institutional Research Board. Subjects were infused with [U-13C]glucose at 8.0 g for 10 minutes as a bolus followed by 8.0 g/h for 2 hours during the surgical removal of the tumor. Resected tumor tissues were immediately snap-frozen and stored at -80°C for further ex vivo NMR analysis. Methods for sampling tumor tissue for NMR spectroscopy have been described previously [4]. 1H-decoupled 13C spectra of tumor extracts were acquired at 150 MHz (for 13C) on an Agilent NMR spectrometer equipped with 3-mm broadband room temperature probe. C2 lactate multiplet signal at 69.21 ppm was used to determine the relative pyruvate recycling flux with respect to glycolysis. NMR data in this report were previously used to quantify the amount of acetyl-CoA derived from glucose metabolism in these patients [4].Results and Discussion
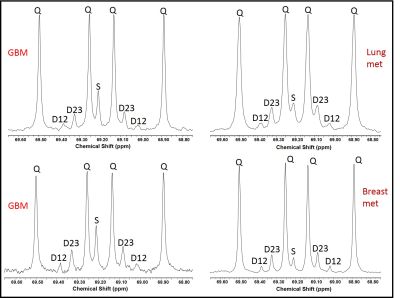
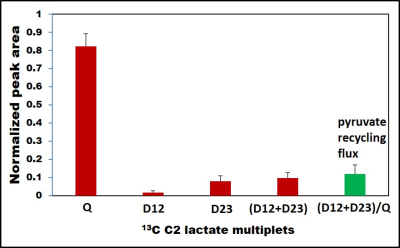
Figure 1 illustrates the pyruvate recycling mechanism during the [U-13C]glucose metabolism. Glycolysis of [U-13C]glucose leads to the generation of [U-13C]pyruvate. From [U-13C]pyruvate, lactate dehydrogenase (LDH) produces [U-13C]lactate and transamination by alanine transferase (ALT) generates [U-13C]alanine. The [U-13C]pyruvate further enters the TCA cycle as [1,2-13C]acetyl-CoA through pyruvate dehydrogenase (PDH) or [1,2,3-13C]OAA via pyruvate carboxylase (PC). Both [1,2-13C] and [3,4-13C]OAA are generated during the first turn of the cycle and [2,3,4-13C]OAA are produced after multiple turns of the cycle. Through the combined activities of phosphoenolpyruvate carboxykinase (PEPCK) and pyruvate kinase (PK), these TCA cycle derived OAA 13C isotopomers are used to produce [1,2-13C], [2,3-13C] and [3-13C]pyruvate and lactate isotopomers. Malic enzyme (ME) can also produce the exact same pyruvate 13C isotopomers from malate 13C isotopomers. [1,2-13C]/[2,3-13C]lactate isotopomers lead to the generation D12 and D23 doublets in C2 lactate 13C NMR spectrum respectively, whereas the quartet (Q) signals arising due to the production of [U-13C]lactate that are generated from glycolysis of [U-13C]glucose (Figure 2). The ratio of the sum of D12 and D23 to Q gives the relative flux of pyruvate recycling with respect to glycolysis. Relative pyruvate cycling flux was found to be at 12.0% ± 5.0% in the brain tumor patients (n=10). Liver and kidney can also generate doubly labeled 13C lactate isotopomers through pyruvate recycling pathway. However, we did not detect any doubly labeled 13C lactate molecules in the circulation which confirms the presence of pyruvate recycling mechanism in the brain tumors.Conclusion
In summary, our data from ten patients suggests that both GBM and brain metastases have active pyruvate recycling pathway in order to meet their elevated bioenergetic requirements.Acknowledgements
This study was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain andPituitary Tumor Treatment Center at Houston Methodist Hospital, The Houston Methodist Foundation, The
Taub Foundation, The Pauline Sterne Wolff Foundation, The Veralan Foundation, The Marilee A. and Gary
M. Schwarz Foundation, The John S. Dunn Foundation and The McKone Family Foundation. We thank all
the patients who participated in this study.
References
1. S. Cerdan, B. Kunnecke, J. Seeling, Cerebral Metabolism of [1,2-13C2]Acetate as detected by in Vivo and in Vitro 13C NMR, J. Biol. Chem. 265 (1990) 12916-12926.
2. S. Cerdan, Twenty-seven Years of Cerebral Pyruvate Recycling, Neurochem Res. 42 (2017) 1621-1628.
3. S. Serres, E. Bezancon, J-M. Franconi, M. Merle, Brain pyruvate recycling and peripheral metabolism: an NMR analysis ex vivo of acetate and glucose metabolism in the rat, J. Neurochem. 101 (2007) 1428-1440.
4. Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR, DeBerardinis RJ. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25 (2012) 1234-44.
Figures