0767
Multi-parametric hyperpolarized 13C/1H imaging of human gliomas expressing diverse pathologic mutations1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, United States, 3Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA, United States
Synopsis
This study uses multi-parametric 1H and hyperpolarized carbon-13 (HP-13C) MRI to characterize patients with gliomas that express signature pathologic mutations at the time of recurrence
Introduction
Gliomas represent a family of brain tumors with tremendous genetic variation that is reflected in their diverse clinical outcomes and imaging characteristics. Given the limitations of conventional imaging, this work takes a multi-parametric approach to investigating such heterogeneity using 1H together with hyperpolarized carbon-13 (HP-13C) MRI, which has shown promise in probing glioma metabolism1-4. We evaluated several patients demonstrating disease recurrence either prior to surgery or as part of routine imaging alongside information regarding signature pathologic mutations.Methods
HP-13C Imaging. Thirteen patients previously diagnosed with and treated for glioma (11 IDH-wildtype/2 IDH-mutant GBM) received multiparametric HP-13C/1H imaging at the time of suspected recurrence (17 total exams). Dynamic HP-13C imaging was performed on a 3T MR750 scanner in combination with a dual-tuned 24/8-channel 13C/1H phased-array receiver (RAPID Biomedical, Germany). Data were acquired using a frequency-specific 2-D multislice EPI sequence5(TR/TE=62.5ms/21.7ms, 8 slices, 20 timepoints, 3s temporal resolution) that featured 1.5 cm isotropic resolution and a constant 𝛼pyruvate/𝛼lactate/𝛼bicarbonate = 20o/30o/30o flip angle scheme. A SPINlab system provided dynamic nuclear polarization of [1-13C]pyruvate, as previously described2. Upon pharmacist release, a 0.43mL/kg dose of [1-13C]pyruvate was injected intravenously at 5mL/s, followed by a 20mL saline flush; imaging began after a 5s delay.1H Imaging. Proton imaging included T2 CUBE FLAIR (TR/TE=5650/86ms); pre/post-Gd T1; 24-direction DWI with b=2000s/mm2; DSC perfusion (TR/TE=1500/25ms); and atlas-based lactate-edited MRSI (TR/TE=1279/144 ms).
Genetic Sequencing. The UCSF500 genetic panel sequenced over 500 cancer-associated genes including isocitrate dehydrogenase (IDH1/2) using a tumor specimen containing >25% malignant cells and non-tumor reference sample. A majority of patients (12/13) had surgical tissue analyzed, with 7 receiving HP-13C/1H imaging immediately prior to surgery.
13C/1H Parameters. 13C EPI data were prewhitened6, channel-combined using complex weights from the fully sampled pyruvate signal7, phased and denoised8. Kinetic modeling of pyruvate-to-lactate conversion (kPL) was performed on dynamic data using an inputless model9. Other parameters were obtained from temporally summed metabolite data normalized by the sine of the respective flip angles. This enabled the calculation of a modified lactate-to-pyruvate ratio, which attempts to remove the vascular component of pyruvate:
$$\frac{nLac} {nPyr [1-\frac{nPyr -(nLac +nBic)}{nPyr + nLac + nBic}]}$$
Imaging data were resampled by a factor of 2 in-plane via linear interpolation. The signal percentiles (%) of individual metabolites within the brain were used to determine relative intensities. Proton imaging parameters included apparent diffusion coefficients (ADC); perfusion peak height (PH), recovery, and cerebral blood volume (CBV); and 1H lactate and choline-to-NAA indices (CNI) from spectroscopy. Normalization by values in normal-appearing white matter was performed for diffusion and perfusion data. Analysis. Voxel-wise analysis evaluated each parameter at the 13C resolution within segmented regions of interest for contrast-enhancing lesion (CEL), non-enhancing lesion (NEL) and normal-appearing white matter (NAWM). Glioma populations were compared using a mixed effects model in R after filtering for SNRcarbon-13 > 10 and kPL error < 30%.
Results
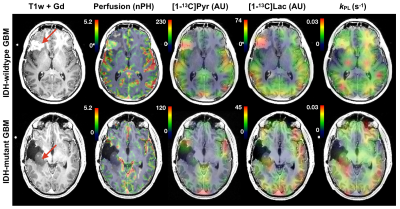
A total of 1849 voxels were analyzed among the patients: 649 NAWM, 990 NEL, and 210 CEL. The following relate the relevant comparisons.IDH-mutant vs IDH-wildtype GBM. [patients(scans): 2(3) vs 9(11)]. IDH-mutant GBM showed lower pyruvate percentiles (p=0.01) and 1H-lactate levels (p=0.04) in the T2 lesion, but higher kPL (p<0.001) and median nADC values (p=0.001). Figure 1 illustrates how kPL can vary between IDH-mutant vs wildtype GBM.
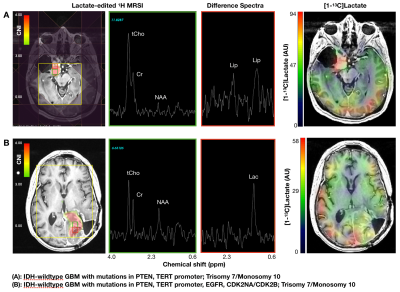
IDH-wildtype GBM. [patients(scans): 9(11)]. Relative to NAWM, the CEL of IDH-wildtype GBM demonstrated higher pyruvate% (p<0.0001), lactate% (p<0.0001), modified ratio (p<0.0002), median nCBV (p=0.008), maxCNI (p=0.0001), 1H-lactate (p<0.0001), higher median nADC; but lower bic/lac (p<0.001); trending higher kPL. The NEL demonstrated higher pyruvate% (p=0.007), lactate percentile (p=0.004), and maxCNI (p<0.0001) compared to NAWM; however, higher bic/lac (p=0.006) and lower nkPL (p=0.04), modified ratio (p=0.0001), pyruvate% (p=0.0001), lactate% (p<0.0001), median CBV (p<0.03), and 1H-lactate (p<0.002) compared to the CEL. Figure 2 illustrates some of the heterogeneity in imaging that may relate to pathologic mutations in GBM, while also describing some of the potential interactions between steady state 1H-lactate and HP [1-13C]lactate.
IDH-wildtype GBM vs Radiation Injury. [patients(scans): 9(11) vs 2(3)]. Two patients with radiation-induced injury resembling IDH-wildtype GBM showed lower 1H-lactate (p=0.03) in the CEL, and lower 1H-lactate (p=0.04) and higher recovery (p<0.001) in the T2-lesion.
Discussion
This work has evaluated multi-parametric 1H/HP-13C imaging from patients at the time of recurrence, as defined by radiological criteria or surgical pathology. Despite the limited number of patient datasets, we managed to observe some interesting variations or patterns that are useful in understanding both the tumor heterogeneity and the underlying methodologies. IDH-mutant GBM can demonstrate higher apparent lactate-to-pyruvate conversion (kPL) than their non-mutated counterparts owing to differences in vascular perfusion that are not easily addressed through kinetic modeling. Importantly, both IDH-mutated and IDH-wildtype GBM displayed evidence of aberrant 1H and HP-13C metabolism that builds upon prior studies1-3. IDH-wildtype GBM, which represent the worst clinical outcomes, displayed considerable heterogeneity with respect to HP parameters and genetics. The lactate-edited 1H spectroscopy provided an interesting comparison between 1H-lactate and HP [1-13C]lactate that may relate to pool size effects; and lower levels of 1H-lactate may help distinguish radiation injury from recurrent tumor.Acknowledgements
NIH Grants R01 CA127612, P01 CA118816, P41 EB013598, P50 CA097257, and T32 CA151022, together with the Glioblastoma Precision Medicine Program.References
1. Autry AW, Gordon JW, Chen, HY, et al. "Characterization of serial hyperpolarized 13C metabolic imaging in patients with glioma", Neuroimage Clinical, 2020; 27:102323.2.
2. Park I, Larson PEZ, Gordon JW, et at. "Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolismin patient studies", Magn Reson Med. 2018; 80(3):864-8733.
3. Miloushev VZ, Granlund KL, Boltyanskiy R, et al. "Metabolic Imaging of the Human Brain with Hyperpolarized C Pyruvate Demonstrates C Lactate Production in Brain Tumor Patients", Cancer Res. 2018; 78(14): 3755-37604.
4. Grist JT, McLean Mary, Riemer Frank, et al. "Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging", NeuroImage 2019, 189: 171-1795.
5. Gordon JW, Vigneron DB, and Larson PEZ, et al. "Development of a Symmetric Echo Planar Imaging Framework for Clinical Translation of Rapid Dynamic Hyperpolarized C Imaging", Magn Reson Med. 2017; 77: 826–8326.
6. Hansen MS. "Parallel Imaging Reconstruction I: Cartesian. Proc. Intl. Soc. Mag. Reson. Med". 18 (2010).
7. Zhu Z, Zhu X, Ohliger M, Cao P, et al. "Coil combination methods for multi-channel hyperpolarized 13C imaging data from human studies", JMR 2019; 301: 73-398.
8. Kim, Yaewon, et al. “Denoising of Hyperpolarized 13C MR Images Using Patch-based Tensor Singular Value Decomposition”, UCSF Department of Radiology Imaging Research Symposium, 20209. Larson, P.E.Z., Chen, H.Y.,
9. Gordon, et al. "Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients," NMR Biomed.,2018: 31 (11), e3997.
Figures