0766
In-vivo Magnetic Resonance Elastography of implanted human prostate tumors in a murine model.1Institute of Radiology, Charité Üniversitätsmedizin, Berlin, Germany, 2School of Medicine & Klinikum Rechts der Isar, Technical University of Munich, Munich (TUM), München, Germany, 3Institut für Medizinische Informatik, Charité Üniversitätsmedizin, Berlin, Germany
Synopsis
Prostate cancer (PCa) is the second leading cause of cancer-death in men in the western world. Advanced techniques of clinical MR-elastography (MRE), allow the characterization of PCa, based on the viscoelastic tissue-properties, which provide rich biophysical signatures of tumor progression. Using multifrequency MRE, we investigated PCa introduced LNCaP cell-lines, in a immunodeficient murine model, in-vivo, in a 3-Tesla MRI-scanner and, ex-vivo, by a 0.5-Tesla compact MRE-device. In-vivo and ex-vivo MRE values of LNCaP were in good agreement given the viscoelastic frequency-dispersion typical for soft-tissues. Compared with patient data in literature, LNCaP in mice are softer than PCa in humans.
Introduction
In the Western world, prostate cancer (PCa) is one of the most frequently diagnosed cancer, and the second leading cause of cancer death in men(1). During the past decades MR-elastography (MRE) was applied for PCa characterization in patients and tissue-samples based on viscoelastic tumor properties(2-10). Viscoelastic parameters of tumors provide rich biophysical signatures of microstructural and histopathological features such as increased cell density and fibrous protein accumulation(4). However, thus far no MRE studies have been performed in mouse models of PCa to further investigate how the viscoelastic-properties of PCa correlate with specific histopathological-features. Therefore, this study reports on preliminary MRE experiments in prostate tumors based on human cell-lines in murine models. To foster translation of the MRE technology, in-vivo experiments were performed in a clinical 3-Tesla MRI scanner. Following these in-vivo examinations, the viscoelastic tumor properties were quantified ex-vivo in a more established compact MRE-scanner operated at 0.5-Tesla. Collectively, this study addresses, for the first time, MRE in a murine model of PCa.Material & Methods
Animal modelAnimal experiments were approved and performed according to the animal-protection-committee (LaGeSo; Berlin, Germany), and the local Guidelines and Provisions for Implementation of the Animal-Welfare-Act by Charité-Universitätsmedizin Berlin. Tumor-cells of the LNCaP cell-line (ATCC, Rockville, USA) were grown in RPMI-1640-medium with 10% fetal-bovine-serum incubated at 37°C and 5% CO2 in air. When a number of 2×106 cells was reached, they were taken up in of RPMI-1640 and transplanted subcutaneously close to the right scapula of 8-10 weeks old male CB17-Prkdscid/IcrCrl mice. Tumors developed in 6 out of 14 animals between 9-17 weeks after implantation to a size of ±1cm3.
MRE examination
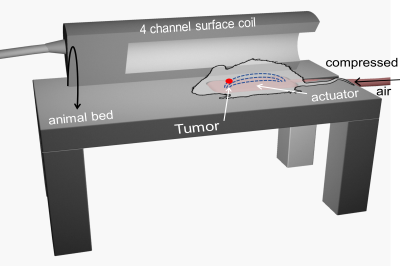
For MR-examinations, the animals were anesthesized by interperitoneal application of 500µg/kg medetomidin, 50µg/kg fentanyl, and 5mg/kg midazolam. MRE was conducted on a clinical 3T-scanner (Magnetom Lumina, Siemens, Germany) with a 4-channel surface-coil (Rapid Biomedical, Rimpar, Germany) dedicated for murine experiments. A standard setup for clinical MRE of the prostate as described in (4) was used. Figure 1 shows the position of an animal on a compressed air-based passive actuator integrated in the animal bed to generate harmonic vibrations at 80-130Hz in10Hz increments. The same type of actuator was used in patients in(4). Full 3D-wavefields were acquired using a single-shot, spin-echo echo-planar imaging sequence with the following parameters: eight phase-offsets over one vibration period, 10 axial slices with 9.2×3.6mm2 field-of-view and 1×1×1mm3 voxel-size, echo-time(TE)=54ms, repetition-time(TR)=2000ms. In-vivo MRE was successfully performed in 5 animals, one animal was excluded due to a corrupted acquisition protocol. Tumors of 3 animals were further investigated ex-vivo at 0.5-Tesla in a compact MRI-scanner(11) at drive-frequencies 600-3200Hz in 400Hz increments. Shear-wave-speed (SWS) maps as a surrogate of stiffness were generated from the complex valued wave images using multifrequency wave number inversion (k-MDEV)(12).
Results
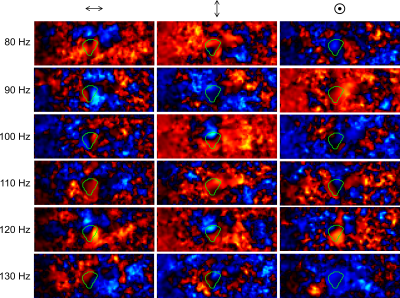
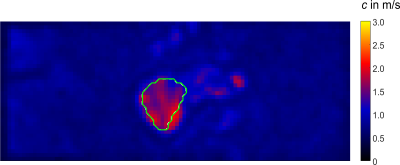
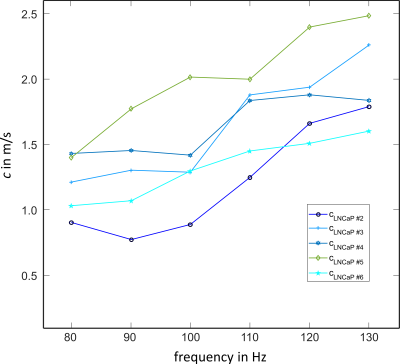
Preprocessed wave-field-components for k-MDEV are depicted in Figure2 for all vibration frequencies for one image-slice. The green line demarcates the tumor boundary. Figure3 shows frequency-averaged (compound) SWS-maps in a central slice of the tumor for all animals. The mean compound-stiffness and standard deviation for the five tumors was 1.47±0.33m/s. Figure4 illustrates the frequency resolved analysis of the data revealing a marked frequency dispersion behavior similar to other soft tissues(13). The SWS determined by compact tabletop MRE, ex-vivo, for three LNCaP tumors for the lowest and highest vibration frequencies 600 and 3200Hz, respectively, are:$$\begin{matrix}\hline c/SWS_{(in\frac{m}{s})} \ |&600Hz&3200Hz\\ \hline LNCaP2|& 2.40& 3.26\\LNCaP3|& 2.26&4.13 \\LNCaP4|&2.2&3.11\\ \hline \end{matrix}$$
Discussion
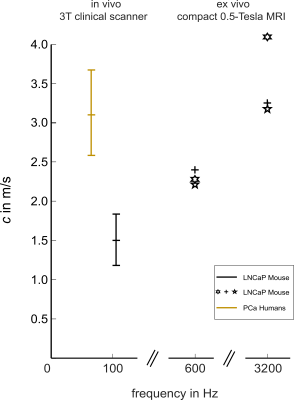
This study shows the feasibility of MRE prostate cancer assessment in a mouse model, screened in a 3-Tesla MRI-scanner (in-vivo) and by a 0.5-Tesla Benchtop MRE-device (ex-vivo). The implanted tumors reached maximum volumetric-diameters of 10mm and, thus, were comparable to tumor sizes observed in humans. The range of applied vibration-frequencies was extended to 130Hz but overlaps, in the lower limit of 80Hz, with the frequency range that is used for clinical examinations.Figure5 summarizes SWS data obtained for LNCaP tumors with data published in (3) for PCa. For clarity, we included only the individual SWS for the start and end frequencies (i.e. 600 and 3200Hz) for ex-vivo data from three LNCaP tumors measured with a Benchtop MRE-scanner(11). Given the frequency dispersion of SWS in soft viscoelastic tissue, there is good agreement comparing the in-vivo and ex-vivo properties from LNCaP tumors. However, LNCaP tumors are apparently softer than PCa investigated in patients, despite the higher frequency-range used in this study. This disparity might point towards the influence of the tissue environment in which a growing tumor is embedded on the tumor’s viscoelastic properties. Further studies in more animals are needed to validate and histologically analyze this interesting observation.
Conclusion
MRE might be an important imaging marker for the aggressivness of PCa that is related to the biophysical properties of the tumor and its environment. Therefore, MRE studies in PCa tumor models in mice are needed which combine in-vivo and ex-vivo investigations along with histological analyses. Our study proposes a setup of MRE in a 3-Tesla clinical MRI-scanner for in-vivo examinations combined with compact 0.5-Tesla MRI experiments for ex-vivo MRE. The obtained preliminary results show that LNCaP tumors grown in mice are softer than in-vivo PCa in patients and are in good agreement between in-vivo and ex-vivo.Acknowledgements
Funding from the German Research Foundation (GRK 2260 BIOQIC, SFB1340 Matrix in Vision) is gratefully acknowledged.References
(1) Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375: 1415–1424.
(2) Arani A, Da Rosa M, Ramsay E, Plewes DB, Haider MA, Chopra R. Incorporating endorectal MR elastography into multi-parametric MRI for prostate cancer imaging: Initial feasibility in volunteers. J Magn Reson Imaging 2013;38:1251-1260.
(3) Arani A, Plewes D, Krieger A, Chopra R. The feasibility of endorectal MR elastography for prostate cancer localization. Magn Reson Med 2011;66:1649-1657.
(4) Asbach P, Ro SR, Aldoj N, Snellings J, Reiter R, Lenk J, Kohlitz T, Haas M, Guo J, Hamm B, Braun J, Sack I. In Vivo Quantification of Water Diffusion, Stiffness, and Tissue Fluidity in Benign Prostatic Hyperplasia and Prostate Cancer. Invest Radiol 2020;55:524-530.
(5) Dittmann F, Reiter R, Guo J, Haas M, Asbach P, Fischer T, Braun J, Sack I. Tomoelastography of the prostate using multifrequency MR elastography and externally placed pressurized-air drivers. Magn Reson Med 2018;79:1325-1333.
(6) Sahebjavaher RS, Baghani A, Honarvar M, Sinkus R, Salcudean SE. Transperineal prostate MR elastography: initial in vivo results. Magn Reson Med 2013;69:411-420.
(7) Sahebjavaher RS, Nir G, Gagnon LO, Ischia J, Jones EC, Chang SD, Yung A, Honarvar M, Fazli L, Goldenberg SL, Rohling R, Sinkus R, Kozlowski P, Salcudean SE. MR elastography and diffusion-weighted imaging of ex vivo prostate cancer: quantitative comparison to histopathology. NMR Biomed 2015;28:89-100.
(8) Sahebjavaher RS, Nir G, Honarvar M, Gagnon LO, Ischia J, Jones EC, Chang SD, Fazli L, Goldenberg SL, Rohling R, Kozlowski P, Sinkus R, Salcudean SE. MR elastography of prostate cancer: quantitative comparison with histopathology and repeatability of methods. NMR Biomed 2015;28:124-139.
(9) Thormer G, Reiss-Zimmermann M, Otto J, Hoffmann KT, Moche M, Garnov N, Kahn T, Busse H. Novel technique for MR elastography of the prostate using a modified standard endorectal coil as actuator. J Magn Reson Imaging 2013;37:1480-1485.
(10) Reiter R, Majumdar S, Kearney S, Kajdacsy-Balla A, Macias V, Crivellaro S, Caldwell B, Abern M, Royston TJ, Klatt D. Prostate cancer assessment using MR elastography of fresh prostatectomy specimens at 9.4 T. Magn Reson Med 2020;84:396-404.
(11) Ipek-Ugay S, Drießle T, Ledwig M, Guo J, Hirsch S, Sack I, Braun J. Tabletop magnetic resonance elastography for the measurement of viscoelastic parameters of small tissue samples. J Magn Reson. 2015 Feb;251:13-8.
(12) Tzschätzsch H, Guo J, Dittmann F, Hirsch S, Barnhill E, Jöhrens K, Braun J, Sack I. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1–10.
(13) Braun J, Tzschatzsch H, Korting C, Ariza de Schellenberger A, Jenderka M, Driessle T, Ledwig M, Sack I. A compact 0.5 T MR elastography device and its application for studying viscoelasticity changes in biological tissues during progressive formalin fixation. Magn Reson Med 2018;79:470-478.
Figures