0765
A Framework for Characterizing Prostate Cancer Heterogeneity Using Voxel-Wise Co-Registered Ex Vivo MRI and Whole-Mount Histopathology1Department of Radiological Sciences, UCLA, Los Angeles, CA, United States, 2Department of Pathology, UCLA, Los Angeles, CA, United States, 3Department of Urology, UCLA, Los Angeles, CA, United States
Synopsis
Tumor heterogeneity is considered a key factor in determining the aggressiveness of prostate cancer (PCa). Quantitative MRI and digital whole-mount histopathology (WMHP) have potential to characterize heterogeneity in PCa in terms of MRI properties and tissue composition, respectively. Therefore, the purpose of this study is to develop a data acquisition and processing framework that produces high-resolution (1x1mm2) co-registered quantitative MRI and digital histopathology maps to enable texture features based quantification of PCa heterogeneity
Introduction
Tumor heterogeneity is considered a key factor in determining the aggressiveness of prostate cancer (PCa)1 and histological tissue changes evaluated by the qualitative Gleason Score (GS) is considered the single most useful marker for PCa prognosis2. The heterogeneity of PCa (spatial, morphological etc.) poses a major challenge to clinical diagnosis of clinically significant (cs) PCa (defined here as GS>3+3) using multi-parametric MRI3.Quantitative MRI and digital whole-mount histopathology (WMHP) have potential to characterize heterogeneity in PCa in terms of MRI properties (e.g., apparent diffusion coefficient [ADC] and quantitative T2) and tissue composition, respectively4-6. Co-registration between the two will enable analysis of voxel-wise relationships between them7, and enable the extraction of quantitative texture features from both MRI and histopathology data in a consistent manner to provide quantitative characterization of tumor heterogeneity.
Therefore, the purpose of this study is to develop a data acquisition and processing framework that produces high-resolution (1x1mm2) co-registered quantitative MRI and digital histopathology maps to enable texture feature-based quantification of PCa heterogeneity.
Methods
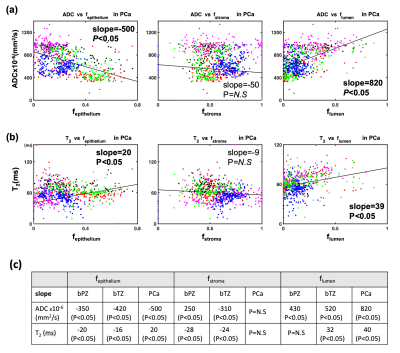
The proposed framework is shown in Figure 1. In an IRB and biosafety-approved study, five fresh whole prostate specimens from PCa patients underwent 3T ex-vivo MRI within 3D-printed molds8. Quantitative MRI maps of ADC (mm2/s) and T2 (ms) were obtained at 1 mm2 resolution8. After MRI, specimens were sectioned in molds to generate co-registered WMHP slides. A pathologist annotated each PCa regions of interest (ROI) on WMHP and reported the GS. For digital histopathology analysis, we used a deep convolutional neural-network to segment the 20x WMHP into epithelium, stroma, and lumen area fraction maps (fepithelium: fE, fstroma: fS, flumen: fL) at 1mm2 resolution9.Voxel-wise correlation between quantitative MRI and digital WMHP maps were evaluated in benign peripheral zone (bPZ), benign transition zone (bTZ) and PCa regions using linear regressions. The voxel-by-voxel quantitative MRI and WMHP parameters were also compared between bPZ, bTZ and PCa with t-tests
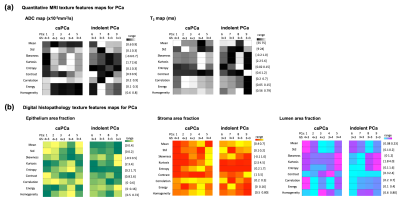
From the co-registered quantitative MRI and histopathology maps, a software tool (Pyradiomics, Boston) was used to calculate first order, histogram, energy, and gray level co-occurrence matrix (GLCM) texture features from PCa ROIs. The trend of texture features variations between PCa of different aggressiveness (e.g. csPCa, GS>3+3 and indolent PCa, GS=3+3), as well as within the same GS were examined for their potential to capture PCa heterogeneity,
Results
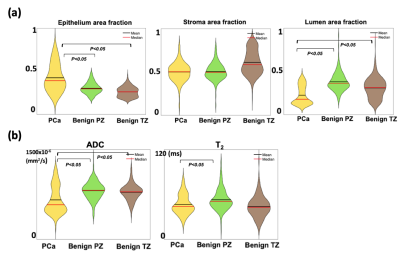
In total, 9100 voxels from bPZ, bTZ and 9 PCa ROIs (4 indolent and 5 csPCa) were analyzed. Significant correlations between quantitative MRI and WMHP parameters were found and varied among tissue types (Fig-2). Voxel-wise WMHP showed PCa had higher fE (P<0.05) and lower fL (P<0.05) than bPZ and bTZ; MRI showed PCa had lower ADC (P<0.05) than bPZ and bTZ, and lower T2 (P<0.05) than bPZ (Fig-3). Texture features obtained from co-registered quantitative MRI and digital histopathology in 9 PCa were shown in Fig-4.Discussions
We used 3D-printed prostate molds and software registration to align quantitative MRI and digital histopathology maps at identical 1x1 mm2 resolution. We are able to generate high resolution, co-registered quantitative MRI and histopathology parameters, and further extracted texture features to characterize PCa spatial heterogeneity. Our preliminary results showed voxel-wise co-registered quantitative MRI and histopathology parameters were correlated within the prostate and showed significant differences between PCa and benign tissues.Comparing csPCa (GS>3+3) to indolent PCa (GS=3+3) in terms of texture features, we observed a trend of lower values of mean, energy and homogeneity and higher entropy in ADC; lower mean, kurtosis and higher correlation and skewness in T2; higher mean, correlation and entropy in epithelium area fraction; lower values of contrast and skewness in stroma area fraction; lower values of correlation, energy and higher kurtosis in lumen area fraction. Variations in terms of features are also observed among PCa of same GS, indicating tissue heterogeneity within same GS group. Texture features from high-resolution MRI and WMHP data may provide additional information for understanding the heterogeneity of PCa and its relationship to cancer aggressiveness.
Conclusions
Using the proposed framework, voxel-wise co-registered quantitative MRI and digital histopathology maps showed correlation with each other and significant differences between benign tissue and prostate cancer. Our framework can enable consistent extraction of texture features from high resolution MRI and digital histopathology data to support further investigations of PCa heterogeneity.Acknowledgements
This work was supported in part by: Integrated Diagnostics Program, Departments of Radiological Sciences and Pathology, David Geffen School of Medicine at UCLA; Jonsson Comprehensive Cancer Center at UCLA; National Cancer Institute of the NIH under award number R01CA248506.References
1. Cyll K, Ersvær E, Vlatkovic L, et al. Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br J Cancer. 2017;117(3):367‐375. doi:10.1038/bjc.2017.171.
2. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. World Health Organization Classification of Tumours.
3. Barrett T, Haider MA. The emerging role of MRI in prostate cancer active surveillance and ongoing challenges. Am. J. Roentgenol. 2017;208:131–139. doi: 10.2214/AJR.16.16355.
4. Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF. Diffusion-weighted MRI of peripheral zone prostate cancer: Comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. Int. Braz J Urol 2010;36:504. doi: 10.1590/S1677-55382010000400018.
5. Dregely I, Margolis DA, Sung K, Zhou Z, Rangwala N, Raman SS and Wu HH. Rapid quantitative T2 mapping of the prostate using threedimensional dual echo steady state MRI at 3T. Magn Reson Med 2016;76:1720–1729
6. Madabhushi A. Digital pathology image analysis: opportunities and challenges. Imaging Med 2009;1(1):7–10.
7. . Kwak JT, Sankineni S, Xu S, et al. Prostate Cancer: A Correlative Study of Multiparametric MR Imaging and Digital Histopathology. Radiology. 2017;285(1):147‐156. doi:10.1148/radiol.2017160906.
8. Wu HH, Priester A, Khoshnoodi P, et al. A System Using Patient-Specific 3D-Printed Molds to Spatially Align In Vivo MRI With Ex Vivo MRI and Whole-Mount Histopathology for Prostate Cancer Research. J. Magn. Reson. Imaging 2019: 49: 270-279. doi:10.1002/jmri.26189.
9. W. Li et al., "Path R-CNN for Prostate Cancer Diagnosis and Gleason Grading of Histological Images," in IEEE Transactions on Medical Imaging, vol. 38, no. 4, pp. 945-954, April 2019, doi: 10.1109/TMI.2018.2875868
Figures