0764
Improving Multiparametric MR - TRUS Guided Fusion Prostate Biopsies with Hyperpolarized 13C Pyruvate Metabolic Imaging : Technical Development1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA, United States
Synopsis
This project developed a new approach of integrating research biopsy targets, defined by metabolic abnormalities on hyperpolarized (HP) 13C pyruvate MRI, into clinical multiparametric MRI workflow to guide software-fusion transrectal ultrasound (TRUS) biopsy for improved prostate cancer risk stratification. Feasibility was investigated in 3 prostate cancer patient studies, for which research biopsy targets corresponding to high pyruvate-to-lactate conversion rates (kPL) were identified and outlined by experienced abdominal radiologists. Fusion biopsies identified histologically-confirmed cancer at two out of three “C13” targets, consistent with clinically low- to intermediate-risk disease, supporting continued active surveillance management for these patients.
Purpose
While conventional multiparametric MRI (mpMRI) is widely used to guide prostate biopsies, its role in the surveillance setting has long been a subject of controversy1,2. Hyperpolarized (HP) 13C-pyruvate MRI can detect significantly different pyruvate-to-lactate conversion rates between clinically significant, aggressive prostate cancer from that of more indolent biology3,4. This technical development study aims to integrate the metabolically-defined research targets based on HP 13C-pyruvate into mpMRI workflow, and guide transrectal ultrasound (TRUS) fusion biopsies to improve the identification of clinically significant disease.Methods
Patient Population: Three patients who were either undergoing or planning to start active surveillance were enrolled (NCT03933670). Key eligibility criteria included presence of biopsy-proven prostate adenocarcinoma in the low- or intermediate-risk group, planned/current enrollment on active surveillance protocol, and ECOG status 0 or 1. The exclusion criteria included prior use of androgen-deprivation therapy, prior prostate radiation treatment, or contraindication for placement of endorectal coils. The clinical risk was stratified based on the UCSF-CAPRA score5.Hyperpolarized-13C MR Exam: Research HP-13C-pyruvate 2-minute acquisitions were integrated into standard-of-care (SOC) prostate mpMRI studies following methods previously described6,7. Briefly, a multi-slice EPI C13 acquisition provided whole gland coverage with 0.33-0.5cc spatial resolution (6.5 - 8mm in-plane resolution, 8mm-thick slices) and 2s temporal resolution, following the injection of GMP HP-[1-13C]pyruvate. Two patients received an optional second 13C-pyruvate injection during the same exam. Pyruvate-to-lactate conversion rate (kPL) maps were calculated for each patient study based on an input-less two-site exchange model8, where they were zero-filled to match the T2w resolution, masqueraded as a “diffusion weighted” series, and pushed to PACS to utilize the built-in color overlay feature on the Dynacad (Philips Invivo, Gainsville FL) analysis/targeting platform.
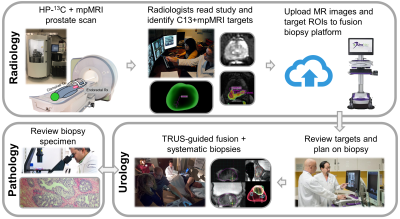
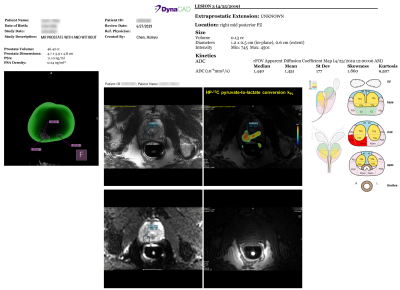
Targeting of 13C Metabolic Lesions and Fusion Biopsy: The workflow is briefly summarized in Figure 1. Board-certified abdominal radiologists, each with 10+ years of experience reading prostate MRI, contoured the C13 targets on the kPL-T2w color overlay displayed on Dynacad, and created a report outlining both SOC mpMRI (PI-RADS9) and kPL research targets for urology review (Figure 2). Subsequently, the mpMRI exams and these targets were uploaded to the fusion biopsy platform (UroNav, Invivo). The C13 targets were defined by the radiologists, following the general rule of having a focal lesion on the kPL map with kPL ≥ 0.02(s-1).
TRUS-guided fusion biopsy procedures were subsequently performed by urological oncologists in conjunction with the systematic biopsies. The mpMRI-defined targets were sampled in addition to the systematic core biopsies per standard-of-care at our institution. The C13 defined targets replaced some systematic cores in the same sextant, in order to not increase the number of biopsies.
Results and Discussions
Three C13-mpMRI studies and the ensuing biopsies were safe and successful without adverse events. The average turnaround time for the MRI report and targeting was less than 3 days after each exam, and can be further expedited when necessary. A sample report (Figure 2) showed the target locations in the 3D segmented prostate, as well as the C13-kPL/T2 overlay, diffusions, and T1-weighted images arranged side by side to assist the urologists planning the biopsies.The patients enrolled in this study had low- to intermediate-risk disease with median age 71 (range:69-72), PSA 8.4 (range:4.2-9.6), and CAPRA score 1 (range:1-3). The median number of targets from proton mpMRI was 1 (range:0-2), and that of 13C MRI defined targets was 1 (range:1). The 13C targets measured 1 cm (range:0.6-1.1) in length, and the median kPL was 0.0325s-1 (range:0.0198-0.0378). All three patients underwent TRUS fusion + systematic biopsies after the integrated mpMRI exam, with 2-3 cores acquired per target.
One patient (Patient 3) had Gleason 3+4 and two patients (Patient 1&2) had 3+3 disease (number of biopsy cores positive for cancer, median: 5/18, range: 4/17-10/18). On a per patient basis, the maximum involvement of any core was 16-56%(median:52%). The cores sampled from 13C targets were Gleason 3+3 in two patients (Patient 1&2) and benign in one patient (Patient 3).
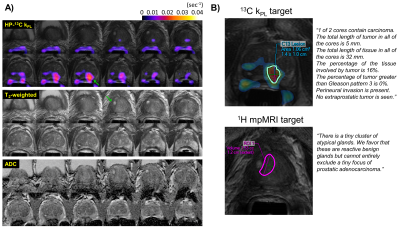
Figure 3 illustrates a representative case (Patient 1) who underwent fusion + systematic biopsies with a 13C MRI defined target (kPL=0.0378s-1) at the left mid-apex peripheral zone, and a 1H mpMRI defined target (PI-RADS 4) at the right mid-base transition zone. Pathology found 3+3 cancer (16% involvement, 1/2 cores) at the 13C target, whereas the 1H mpMRI target consisted of rare atypical glands. Systematic biopsy found 3/12 cores with low volume 3+3 disease. Altogether, Patient 1 had CAPRA score of 1, consistent with low-risk features.
Taken together with other clinical biomarkers, the biopsy findings in the three patients were consistent with clinically low- to intermediate-risk disease (summarized in Figure 4 table), indicating that these patients are appropriate candidates for active surveillance. All patients continued on active surveillance after the study.
Conclusions
This technical development study demonstrated the feasibility of diagnostic intervention using HP-13C pyruvate MRI. HP-MRI was integrated into the diagnostic mpMRI workflow, complete with identification of C13 research targets and sampling of these targets in fusion biopsies. These initial results supported an ongoing study in a larger cohort of patients to evaluate the role of HP-13C MRI + targeted biopsy for improving PCa risk stratification.Acknowledgements
This work was supported by grants from the NIH (U01CA232320, U01EB026412, and P41EB013598).References
[1] Ma, TM et al., The Role of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Biopsy in Active Surveillance. Eur Urol 2017; 81(2), 174-180.
[2] Stavrinides V et al., MRI in active surveillance: a critical review. Prostate Cancer Prostatic Dis 2018; 22(1), 5-15.
[3] Nelson SJ et al., Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med 2013; 5(198), 198ra108.
[4] Granlund KL et al., Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab 2020; 31(1), 105-114.
[5] Cooperberg MR et al., The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 2005; 173(6), 1938-42.
[6] Chen HY et al., Technique development of 3D dynamic CS‐EPSI for hyperpolarized 13C pyruvate MR molecular imaging of human prostate cancer. Magn Reson Med 2018; 80(5), 2062-2072.
[7] Gordon JW et al., Translation of Carbon‐13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med 2019; 81(4), 2702-2709.
[8] Larson PEZ et al., Investigation of analysis methods for hyperpolarized 13C‐pyruvate metabolic MRI in prostate cancer patients. NMR Biomed 2018; 31(11), e3997.
[9] Turkbey B et al., Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019; 76(3), 340-351.
Figures