0757
MOdel-free Diffusion-wEighted MRI (MODEM) with Machine Learning for Cervical Cancer Detection1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Department of Radiology, Tongji Hospital, Wuhan, China, 4Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Characterization of diffusion-weighted imaging signal is typically performed by modeling the data based on biophysical, mathematical, and/or statistical models to estimate quantitative biomarkers. However, conventional nonlinear fitting, which is required for the estimation of model parameters, often suffers from instability and degeneracy. In this study, we propose a Model-free Diffusion-wEighted MRI technique (MODEM) with machine learning to detect cervical carcinomas by using diffusion signal intensities and the first-order statistical features extracted from the signal attenuation as the input. By using MODEM, superior diagnostic performance and stability can be achieved even with limited number of b-values in cervical cancer detection.
Introduction
In the past two decades, diffusion-weighted imaging (DWI) has been increasingly used in routine clinical MRI protocols for cancer detection due to its ability to identify lesions that would otherwise be missed by conventional MRI sequences.1-4 The decay pattern of the diffusion-weighted signal contains valuable information regarding diffusion properties of the water molecules in different tissue microenvironments. Classically, the relationship between the diffusion-weighted signal and the underlying tissue microstructures is retrieved by fitting the signal attenuation to biophysical, mathematical, and/or empirical diffusion models.5-9 However, application of these models typically involves traditional computational algorithms, such as nonlinear least squares fitting. These algorithms often require the acquisition of a set of images with a large number of b-values, inevitably resulting in long acquisition times. Recently, several studies indicated that the conventional model fitting is prone to instability and degeneracy even when q-space is highly oversampled.9,10 The goal of this study is to 1) employ a machine learning-based approach to develop a MOdel-free Diffusion-wEighted MRI technique, MODEM, to detect cervical carcinomas based on diffusion signal attenuation signatures; 2) investigate the feasibility of MODEM in shortening the acquisition time by achieving the same diagnostic outcome with a reduced number of b-values.Materials and Methods
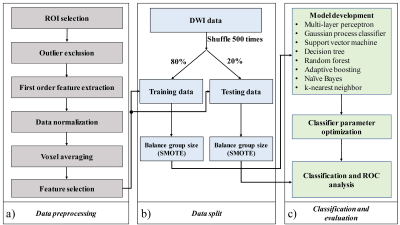
DWI was performed on fifty-four histopathologically confirmed cervical cancer patients on a 3T MRI scanner (Discovery MR750; GE Healthcare) with 15 b-values from 0 to 3600 sec/mm2. To investigate the feasibility of using MODEM to reduce scan times, four different sub-datasets, consisting of different b-value ranges were created: 1) “full” dataset with all 14 non-zero b-values, 2) reduced low-b-value dataset, 3) reduced mid-b-value dataset, and 4) reduced high-b-value dataset, with the last three sub-datasets containing 5 b-values in the ranges of [50 to 1000 sec/mm2], [500 to 1700 sec/mm2], and [1300 to 3600 sec/mm2], respectively. The corresponding acquisition times of these four sub-datasets were 6min 9s, 1min 9s, 2min 16s, and 4 min 16s, respectively. Data pre-processing was performed as illustrated in Fig. 1a. Regions of interest (ROIs) were placed on the tumor and normal uterus tissue. Signal intensity features (normalized signal intensities at each non-zero b-value), along with 10 first-order histogram features (mean, variance, kurtosis, skewness, entropy, range, coefficient of variation (CV), geometric mean, uniformity and root mean square (RMS)) were computed from the signal decay signatures. By using Welch’s t-test, features with p-value > 0.05 were considered insignificant, thus excluded from the input to MODEM. A data split procedure was used to split and augment the samples, as shown in Fig. 1b. The ROIs were split randomly, and stratified into training (80%) and testing sets (20%). A synthetic minority oversampling technique (SMOTE) was performed to generate samples from the minority group to balance the size of the majority group in training and testing data, respectively. Classification and evaluation are illustrated in Fig. 1c. Eight machine learning classifiers were implemented using Python Scikit-learn. Mean and standard deviation values for sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) from each machine learning classifier on the testing dataset were obtained through repeating the analysis steps for data split and classification and evaluation with 500 iterations.Results
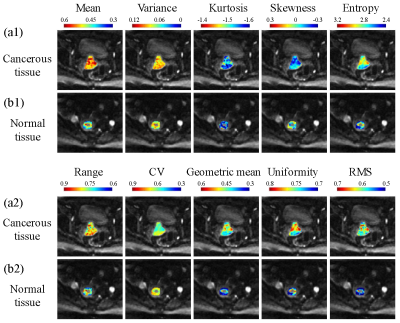
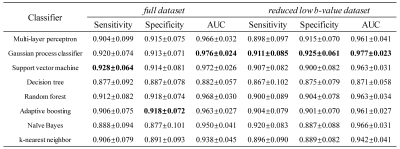
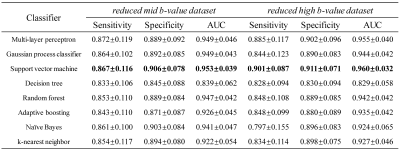
The first-order statistical feature maps of cancerous and normal tissue from a representative patient are shown in Fig. 2. Compared to the cancerous tissue (a1 and a2), the normal tissue (b1 and b2) exhibited lower mean signal intensity, variance, entropy, range, geometric mean, uniformity, and RMS, while yielding higher range and CV. The value of kurtosis was similar between the cancerous and normal tissues. The diagnostic performances of using the full and reduced datasets for the detection of cancerous tissue using eight different machine learning classifiers are listed in Fig. 3 and Fig. 4. In the full dataset experiment, Gaussian process classifier exhibited the highest AUC (0.976) with the corresponding sensitivity and specificity of 0.920 and 0.913, respectively. In the reduced low b-value dataset experiment, Gaussian process classifier outperformed the other classifiers with an AUC of 0.977, sensitivity of 0.911, and specificity of 0.925. In the reduced mid- and high-b-value datasets, support vector machine yielded the highest sensitivity (0.867 and 0.901), specificity (0.906 and 0.911), and AUC (0.953 and 0.960). Reduced low-b-value dataset displayed similar overall diagnostic performances for detecting cancerous tissues with the full dataset, slightly better than the reduced mid-b-value and reduced high-b-value datasets. Overall, the AUCs of the different machine learning classifiers exhibited excellent stability with standard deviations within 0.1 in all datasets.Discussion and Conclusion
In this study, we demonstrated that a model free machine-learning approach for analyzing diffusion signal decay signatures can detect cervical cancerous tissues with high accuracy. The reduced low b-value datasets yielded a similar overall diagnostic performance for detecting cancerous tissues to the full dataset, indicating that MODEM can help reduce the scan times from 6min 9s to 1min 9s without compromising the diagnostic performance. DWI with low b-values can also relax the requirement for strong gradient strength and improved the signal-to-noise ratio. These features, together with the high stability, suggest that MODEM can lead to automatic detection of cervical cancer using machine learning without requiring diffusion-weighted images with high b-values. Our results may be further expanded to detecting other cancers.Acknowledgements
No acknowledgement found.References
[1] Murata T, Shiga Y, Higano S, et al. Conspicuity and evolution of lesions in Creutzfeldt-Jakob disease at diffusion-weighted imaging. Am. J. Neuroradiol. 2002;23(7):1164-72.
[2] Eiber M, Holzapfel K, Ganter C, et al. Whole‐body MRI including diffusion‐weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J. Magn. Reason. Imaging. 2011;33(5):1160-70.
[3] Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125.
[4] Tang L, Zhou XJ. Diffusion MRI of cancer: From low to high b‐values. Journal of Magnetic Resonance Imaging. 2019 Jan;49(1):23-40.
[5] Zhou XJ, Gao Q, Abdullah O, et al. Studies of anomalous diffusion in the human brain using fractional order calculus. Magn Reson Med. 2010; 63(3):562-569.
[6] Karaman MM, Sui Y, Wang H, et al. Differentiating low‐and high‐grade pediatric brain tumors using a continuous‐time random‐walk diffusion model at high b‐values. Magn Reson Med. 2016;76(4):1149-1157.
[7] Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497-505.
[8] Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-1440.
[9] Novikov DS, Kiselev VG, Jespersen SN. On modeling. Magn Reson Med. 2018;79(6):3172-3193.
[10] Jelescu IO, Veraart J, Fieremans E, et al. Degeneracy in model parameter estimation for multi‐compartmental diffusion in neuronal tissue. NMR Biomed. 2016;29(1):33-47.
Figures