0756
Left Ventricular Distribution of LGE and Intra-myocardial Fat in Boys with Duchenne Muscular Dystrophy and Healthy Controls at 3T1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Medicine (Pediatric Cardiology), Children's Hospital of Orange County, Orange, CA, United States, 4Pediatrics (Cardiology), University of California, Los Angeles, Los Angeles, CA, United States, 5Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 6Radiology, Stanford University, Stanford, CA, United States, 7Cardiovascular Institute, Stanford University, Stanford, CA, United States, 8Maternal and Child Health Research Institute, Stanford University, Stanford, CA, United States
Synopsis
Duchenne muscular dystrophy (DMD) – a fatal X-linked genetic disorder – is characterized by progressive muscle weakness, pediatric onset cardiomyopathy, and ultimately heart failure. LGE imaging is the gold standard for detecting myocardial replacement fibrosis, but not fatty infiltration. We used chemical-shift based water-fat separation MRI techniques to investigate myocardial fibro-fatty infiltration and identify the onset of microstructural remodeling in boys with DMD. This study aimed to: 1) report and compare the left-ventricular (LV) intra-myocardial fat content in boys with DMD and healthy controls; and 2) determine whether fatty infiltration precedes the appearance of LGE in boys with DMD.
Introduction
Cardiovascular disease is the leading cause of death in patients with Duchenne muscular dystrophy (DMD) – a fatal X-linked genetic disorder characterized by progressive muscle weakness and pediatric onset cardiomyopathy1-3. Sensitive cardiac MRI biomarkers, including myocardial fibro-fatty infiltration, may help to identify the on-set of microstructural remodeling in DMD. Histologic studies of autopsy hearts from patients with DMD are indicative of fatty infiltration, which is described as “predominantly epimyocardial” 4. Conventional late gadolinium enhancement (LGE) imaging is the current gold standard for detecting myocardial replacement fibrosis, but not fatty infiltration1,2,4. The use of chemical-shift based fat-water separation MRI techniques5 may help to further distinguish fat-infiltrated tissue from replacement fibrotic tissue detected by LGE. This 3T MRI study aims to: 1) report and compare the left-ventricular (LV) intra-myocardial fat content in boys with DMD and healthy controls; and 2) determine whether fatty infiltration precedes the appearance of LGE in boys with DMD.Methods
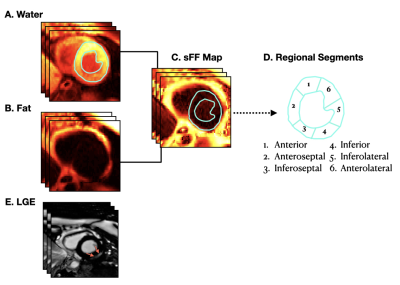
Imaging: Boys with DMD (N=20, 13±2.9 yo) and healthy age- and sex-matched controls (N=11,13.2±2.1 yo) were prospectively enrolled (IRB-approved, informed consent) and underwent a 3T (Skyra, Siemens) cardiac MRI (cMRI) exam. The cMRI exam included standard functional imaging, and a multi-echo (Dixon-type) GRE sequence for fat-water-separated imaging5 after LGE imaging. Multi-slice, short-axis (SA) datasets spanning from base to apex in the LV were acquired during free-breathing with ECG gating. Typical imaging parameters were: matrix size = 192x144mm2, voxel size=2x2x8mm3, flip angle=12,TE/TR=1.64/4.17/6.7/9.23/11.2ms.Post-processing: Two expert clinicians performed the functional image analysis and noted the presence or absence of segmental LGE according to the AHA 17-segment model6. A patient with LGE in at least one myocardial segment was considered to be LGE positive (LGE +). If no enhancement was observed, then the patient was LGE negative (LGE−). Water- and fat-separated images for all short axis slices were used to calculate signal fat fraction maps (Figure 1), sFF=Fat/(Water+Fat) (MATLAB, MathWorks). To account for noise bias, the sFF in regions of low fat content were generated by: sFF=1–sWF (sWF=Water/(Water+Fat). In each slice, a region of interest (ROI) encompassing the LV myocardium was segmented, extracted, and analyzed for their corresponding global and segmental sFF values (Figure 1). Summary statistics were extracted from all ROIs and compared between LGE+ and LGE- boys with DMD and healthy controls. Group-wise comparisons were performed using a Wilcoxon-rank sum test. Data is reported as median and inter-quartile range (IQR). A P-value<0.05 was considered significant.
Results
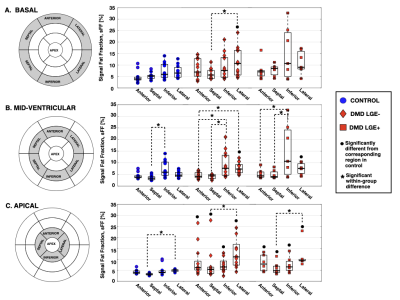
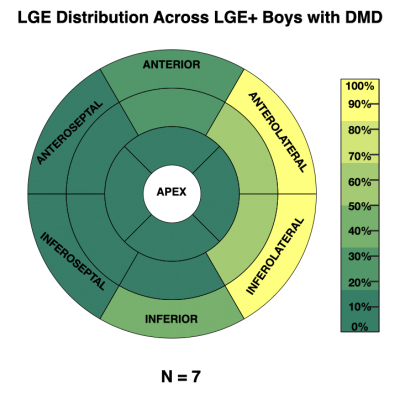
7 (35%) of the boys with DMD were LGE+. Figure 2 shows the distribution of LGE+ segments. All LGE+ boys with DMD had at least one lateral LGE+ segment. Affected segments occurred more frequently in the anterolateral (33%) and inferolateral (33%) LV wall, whereas the septum (6.3%) was less frequently affected. Figure 3C shows the distribution of sFF values in LGE+ boys with DMD. Similarly, increased sFF values are observed in LGE+ segments, but there is more variability across the segments. Figures 3 and 4 characterize the distribution of sFF in the three groups. The variability in sFF values increases with disease progression. Compared to controls, LGE- boys with DMD showed significantly increased lateral sFF at the base [10.6(10.2)% v. 6.5(4.4)%; p=0.04], mid-ventricle [7.3(3.6)% v. 4.6(1.7)%; p=0.003], and apex [11.1(9.5)% v. 5.1(0.8)%; p<0.01]. At the apical level only, anterior [6.2(4.1)% v 3.9(1.2)%; p=0.01], septal [5.4(2.9)% v. 3.2(0.6)%; p=0.002], and inferior [6.5(3.9)% v. 4.0(0.9)%; p=0.02] sFF values are significantly increased in LGE- boys with DMD compared to healthy controls. This trend is also observed in the comparison between LGE+ boys with DMD and healthy controls at the apical level (Figure 4C). LGE+ boys also show increased lateral sFF [7.6(4.2)% v. 4.6(1.7)%; p=0.03] compared to controls at the midventricular level.Discussion
We note non-zero (~5-10%) sFF values in the healthy, age- and sex-matched control group, thus warranting further investigation into the normal levels of intra-myocardial fat in pediatric subjects. While the distribution of sFF in healthy controls shows the least variability, we observe increased sFF in the lateral LV, relative to the septal LV. The reported values help to establish reference sFF values for healthy boys at 3T. We detect increased sFF values in LGE- boys with DMD compared to healthy controls. This finding suggests that: 1) fatty-infiltration can be detected before the appearance of LGE; and 2) fat-water-separated MRI may be more sensitive to the myocardial inflammatory cascade7, which precedes myocardial fibrosis. As regards LGE+ boys with DMD, the pattern of affected myocardial segments is consistent with previous studies noting that LGE findings predominately appear the lateral LV wall in DMD8-10. Similarly, increased sFF values are observed in LGE+ segments, but there is greater variability across the segments (Figures 2-3). Furthermore, we show that this heterogeneity in sFF measurements increases with disease progression (Figures 3-4).Conclusion
Herein, we report increased regional sFF values in LGE+ and LGE- boys with DMD compared to healthy, age- and sex-matched controls. Additionally, we report increased sFF values predominantly in the lateral LV wall – a region most frequently exhibiting LGE+ findings at later stages of cardiovascular disease in DMD.Acknowledgements
We acknowledge the scientific contributions of Patrick Magrath, the pulse sequence support from Peter Kellman, and funding: NIH R01 HL131975 to DBE and NSF DGE 1650604 to NGM.References
[1] Ryder S et al., The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79.
[2] D'Amario D et al., A current approach to heart failure in Duchenne muscular dystrophy. Heart. 2017;103(22):1770.
[3] Florian A et al., Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging. 2014;15(0):1004-12.
[4] Frankel KA et al., The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7(4):375-86.
[5] Kellman et al., Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn Reson Med. 2009;61(1):215-21.
[6] Cerqueira MD et al., Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation. 2002;105(4):539-42.
[7] Tsuda T et al., Dystrophic Cardiomyopathy: Complex Pathobiological Processes to Generate Clinical Phenotype Cardiovasc Dev Dis. 2017;4(3).
[8] Hor KN et al., Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 2013;107(15).
[9] Florian A et al., Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;81(16).
[10] Ganigara M et al., Unique pattern of late gadolinium enhancement on cardiac magnetic resonance imaging in Duchenne muscular dystrophy. Ann Pediatr Cardiol. 2016;9(2):190-191.
Figures
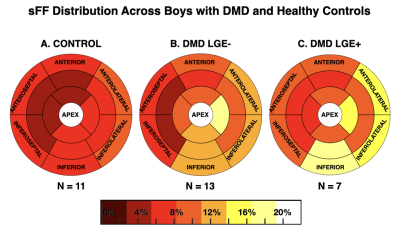
Left-ventricular distribution of signal fat fraction (sFF) in (A) healthy contols; (B) LGE- and (C) LGE+ boys with DMD. Increased sFF values are observed in LGE+ segments, but there is more variability across the segments. Compared to healthy controls, increased sFF values are also observed in LGE- boys with DMD.