0755
Whole-Heart Motion-Resolved Multi-Peak Fat-Fraction Mapping using Free-Running 3D Radial Multi-Echo GRE and Pilot Tone1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland
Synopsis
A free-running multi-echo GRE approach was proposed for whole-heart fat quantification. Retrospective extraction of cardiac and respiratory motion states was achieved using integrated Pilot Tone navigation, enabling a free-breathing non-ECG-triggered acquisition. Following a motion-resolved compressed sensing based image reconstruction of the separate echoes, fat fraction, water fraction, R2* and B0 maps, as well as separated fat and water images, were calculated. Free-running acquisition parameters were optimized in a fat phantom. Volunteer experiments demonstrated the feasibility of motion-resolved free-running fat-fraction mapping technique in a 6-minute scan time.
Introduction
Noninvasive methods for cardiac fat quantification may aid diagnosis in patients suffering from myocardial fat infiltration[1,2], dilated cardiomyopathy[3,4], and help characterize the role of intramyocardial fat in obesity and diabetes[5,6]. Gold-standard fat-fraction (FF) maps are currently obtained using multi-echo gradient echo (ME-GRE) acquisitions followed by maximum-likelihood fitting[7,8,9,10]. Accurate quantification exploits multi-peak fat models[11,12] (Npeaks≥6), which can theoretically only be resolved with a minimum number of NTE=Npeaks+2 acquired echoes[9,12]. In cardiac MRI, respiratory motion is often addressed using breath-holds, while ECG triggering and the selection of a quiescent acquisition window helps avoid cardiac motion blurring[13]. Recent developments in free-running cardiac MRI[14,15], with integrated Pilot Tone (PT)[16,17,18,19], make it possible to retrospectively compensate for cardiac and respiratory motion. Free-running developments may benefit FF mapping because 1) an uninterrupted acquisition poses no limits on the amount of echoes, 2) removes the need for external triggering devices or breath-holds and improves ease of use and patient comfort, 3) provides whole-heart coverage, and 4) may simultaneously provide functional information such as ejection fraction. The aim of the study was to propose a novel free-running 3D radial ME-GRE approach for whole-heart fat-fraction mapping, with integrated PT navigation for cardiac and respiratory signal gating. The sequence was optimized in a phantom with fat components. Proof of concept motion-resolved free-running fat-fraction mapping was tested in healthy volunteers.Methods
All experiments were performed on a 1.5T clinical scanner (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany) using a 12-channel body coil equipped with a PT transmitter.Phantom experiments
The prototype free-running 3D radial ME-GRE sequence incorporated a readout of NTE=8 echoes at each segment of the spiral (Fig.1). In a fat phantom (Fig.2), the effect of receiver bandwidths and RF excitation angles on the measured fat fraction was tested. Sequence parameter ranges that were tested included RF excitation angles [5;25]°, bandwidths [321;1063]Hz/pixel, monopolar or bipolar readout gradients, and echo spacings [1.29;3.65]ms. Fat fractions estimated with graph cut maximum-likelihood fitting[7] were compared to values obtained with MR spectroscopy (MRS) (Fig.2).
Volunteer experiments
Free-running ME-GRE was performed in n=5 healthy volunteers after providing informed written consent. Optimized sequence parameters included: (2.0mm)3 spatial resolution, receiver bandwidth of 893Hz/pixel, RF excitation angle 12°, a monopolar readout gradient, NTE=8, echo spacing TE=2.05ms, TE1=1.25ms. The acquisition time was 6:15min per subject. Cardiac and respiratory signals were retrospectively extracted from PT and used to bin acquired k-space data into 2 respiratory and 10-11 cardiac motion states[15,19]. A compressed sensing reconstruction (similar to that reported in [15] and based on [20,21]) of our 6-dimensional (x-y-z-cardiac-respiratory-echo) data was used to provide motion-resolved 3D volumes of each echo. Using a 6-peak fat-model and a maximum-likelihood graph cut fitting algorithm[7] (ISMRM Fat/Water Toolbox), fat fraction, R2*, B0 maps and fat-only and water-only images were reconstructed (Fig.1). The influence on fat quantification of the selection of cardiac phase and number of echoes used for the fitting was determined in regions of interest (ROI) placed in chest and pericardial fat. Statistical significance was determined using a paired Student’s t-test.
Results
Phantom experimentsFat quantification was not bandwidth-dependent in our examined range, despite the increase in noise. The free-running ME-GRE sequence parameters yielding the most similar FF estimation with respect to MRS were selected for volunteer experiments, and resulted in fat-fractions with a coefficient of determination of r2=0.9885 for a slope with 95% confidence interval in [0.7588;1.026] (Fig.2).
Volunteer experiments
PT allowed the successful extraction of motion signal in all volunteers, which enabled motion-resolved reconstructed fat-images, water-images, R2* maps, and FF maps (Fig.3). The analysis of FF maps obtained at different phases of the cardiac cycle (Fig.4) revealed a consistent estimation in chest fat fraction yet a decrease in the pericardium during systole. The comparative analysis of maps obtained using four or eight echoes (Fig.5) yielded FF maps of similar quality. Quantitatively, while a significant difference (p=0.0252) was observed in the chest fat, the same analysis in pericardial fat showed no difference (p=0.6096). The quality of R2* maps decreased with fewer echoes.
Discussion
Although GRE typically requires a low receiver bandwidth to mitigate noise, FF quantification was not affected by increasing the receiver bandwidth to 893Hz/pixel, which benefits both shorter acquisition time as well as echo spacing. The combined use of PT and the free-running approach to cardiac MRI simplifies access to whole-heart fat quantification, as it allows to retrospectively select a cardiac phase and to account for RR fluctuations. It remains to be investigated whether the observed variation in pericardial FF was due to errors in acquisition/reconstruction/ROI selection, or if it indicates a physiological effect. Although the use of four or eight echoes did not significantly affect the quantification of FF in volunteers, an increased number of echoes would certainly benefit the quantification of lower fat fractions, as more peaks in the fat spectrum could be resolved.Conclusion
A novel free-running 3D radial ME-GRE acquisition and reconstruction approach was developed with integrated PT navigation that provided motion-resolved whole-heart fat-fraction maps. Free-running ME-GRE neither relies on ECG triggering nor on breath-holds and allows the acquisition of an arbitrary number of echoes within a fixed scan time.Acknowledgements
We acknowledge the use of the Fat‐Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm) for some of the results shown in this article.
This study was supported by the Swiss National Science Foundation (PZ00P3_167871 and PCEFP2_194296), the Swiss Heart Foundation (FF18054), the UNIL Bourse Pro Femmes, and the Emma Muschamp Foundation.
References
[1] Mordi, Ify, et al. "Prevalence and prognostic significance of lipomatous metaplasia in patients with prior myocardial infarction." JACC: Cardiovascular Imaging 8.9 (2015): 1111-1112.
[2] Kellman, Peter, et al. "Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 61.1 (2009): 215-221.
[3] Lu, Minjie, et al. "Fat deposition in dilated cardiomyopathy assessed by CMR." JACC: Cardiovascular Imaging 6.8 (2013): 889-898.
[4] Kellman, Peter, Diego Hernando, and Andrew E. Arai. "Myocardial fat imaging." Current cardiovascular imaging reports 3.2 (2010): 83-91.
[5] McGavock, Jonathan M., et al. "Adiposity of the heart*, revisited." Annals of internal medicine 144.7 (2006): 517-524.
[6] Sironi, A. M., et al. "Impact of increased visceral and cardiac fat on cardiometabolic risk and disease." Diabetic Medicine 29.5 (2012): 622-627.
[7] Hernando, Diego, et al. "Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 63.1 (2010): 79-90.
[8] Caussy, Cyrielle, et al. "Optimal threshold of controlled attenuation parameter with MRI‐PDFF as the gold standard for the detection of hepatic steatosis." Hepatology 67.4 (2018): 1348-1359.
[9] Reeder, Scott B., et al. "Multicoil Dixon chemical species separation with an iterative least‐squares estimation method." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 51.1 (2004): 35-45.
[10] Yu, Huanzhou, et al. "Multiecho reconstruction for simultaneous water‐fat decomposition and T2* estimation." Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 26.4 (2007): 1153-1161.
[11] Reeder, Scott B., et al. "Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling." Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 29.6 (2009): 1332-1339.
[12] Yu, Huanzhou, et al. "Multiecho water‐fat separation and simultaneous R estimation with multifrequency fat spectrum modeling." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 60.5 (2008): 1122-1134.
[13] Jaubert, Olivier, et al. "T1, T2, and Fat Fraction Cardiac MR Fingerprinting: Preliminary Clinical Evaluation." Journal of Magnetic Resonance Imaging (2020).
[14] Coppo, Simone, et al. "Free‐running 4D whole‐heart self‐navigated golden angle MRI: initial results." Magnetic resonance in medicine 74.5 (2015): 1306-1316.
[15] Di Sopra, Lorenzo, et al. "An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI." Magnetic resonance in medicine 82.6 (2019): 2118-2132.
[16] Speier, P., M. Fenchel, and R. Rehner. "PT‐Nav: a novel respiratory navigation method for continuous acquisitions based on modulation of a pilot tone in the MR‐receiver." Proc European Society for Magnetic Resonance in Medicine and Biology 32 (2015): 128.
[17] Bacher, M. "Retrospective Evaluation of Pilot Tone Based Cardiac Trigger Quality In A Volunteer Cohort." Book of Abstracts ESMRMB 30 (2017): 360-361.
[18] Schroeder, L., et al. "Two‐dimensional respiratory‐motion characterization for continuous MR measurements using pilot tone navigation." Proceedings of the 24th Annual Meeting of ISMRM, Singapore. 2016.
[19] Falcão, Mariana BL, et al. "5D Flow–A quantitative in vivo comparison between Self-Gating and Pilot Tone Gating."
[20] Feng, L. I., et al. "5D whole‐heart sparse MRI." Magnetic resonance in medicine 79.2 (2018): 826-838.
[21] Sun, Jian, Huibin Li, and Zongben Xu. "Deep ADMM-Net for compressive sensing MRI." Advances in neural information processing systems. 2016.
[22] Piccini, Davide, et al. "Spiral phyllotaxis: the
natural way to construct a 3D radial trajectory in MRI." Magnetic
resonance in medicine 66.4 (2011): 1049-1056.
Figures
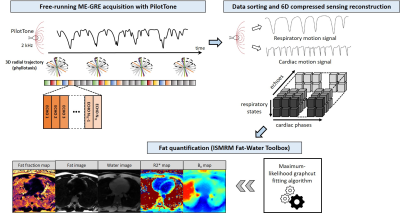
Fig.1 Prototype acquisition, reconstruction and post-processing framework
The uninterrupted whole-heart 3D radial free-running ME-GRE acquisition contains 1000 radial interleaves rotated by the golden angle[22]. Each segment of the spiral is repeated for NTE echoes. The PT signals are used to retrospectively assign acquired segments to specific cardiac and respiratory motion states. After motion-resolved image reconstruction with XD-GRASP-ADMM[20,21], an N-peak fat spectrum model and graph cut algorithm[7] are used to obtain FF, R2* and B0 maps, and fat and water images.
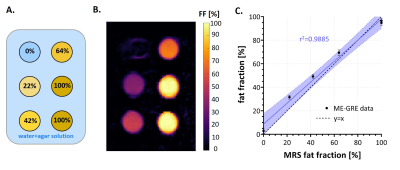
Fig.2 Phantom experiment
A custom-built fat phantom made with peanut oil, agar, and water (A) was used to test the proposed free-running ME-GRE sequence. The fat-fraction map obtained (B) with the maximum-likelihood fitting routine[7] shows good agreement with the gold-standard MRS estimation (C). The shaded blue area represents the 95% confidence interval on the regression line.
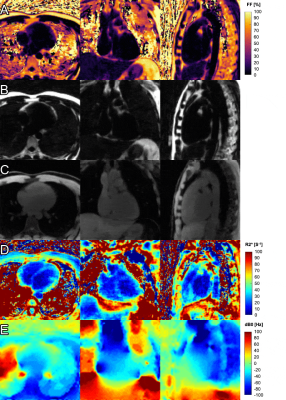
Fig.3 Motion-resolved maps and images of the whole heart in transversal, coronal and sagittal view in one healthy volunteer (animated gifs)
A. Fat fraction map
B. Fat-only image
C. Water-only image
D. R2* map
E. dB0 map (deviation from main field strength B0)
Animated gifs loop through the 11 different phases of the cardiac cycle. Slices displayed in rows A, B, and C were chosen to highlight fatty regions of the heart. Slices displayed in rows D and E were chosen to highlight blood/myocardium contrast in the R2* maps and therefore do not match the slices displayed in rows A, B and C.
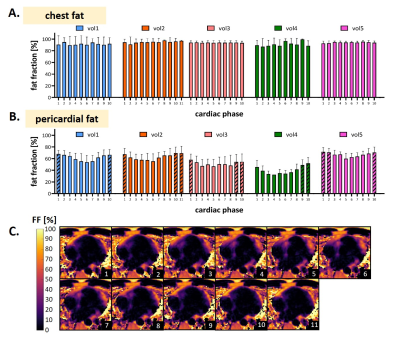
Fig.4 Fat fraction measurements across the cardiac cycle
A. In static chest fat, the fat fraction estimation across the cardiac cycle has little to no variation, across volunteers.
B. In pericardial fat, a lower fat fraction is measured during the cardiac states identified as systole, with respect to those identified as corresponding to the resting phase of the heart, the latter being indicated by a diagonal pattern.
C. Transversal view of fat fraction maps in 11 cardiac phases, in a healthy volunteer (vol2).
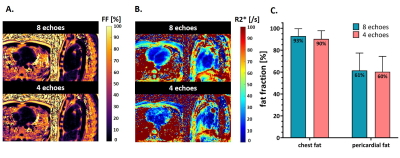
Fig.5 Comparison of mapping results with 8 and 4 echoes
A. Fat fraction maps obtained with 8 and 4 echoes are visually consistent.
B. R2* maps obtained with 4 echoes are noisier than those obtained with 8 echoes, altering the visualization of anatomy (myocardium).
C. Mean estimated fat fraction and standard deviation measured across 5 volunteers, in ROIs selected in the chest fat and in pericardial fat, obtained with the two methods.