0754
MP-Dixon-GRASP: Magnetization-Prepared Multiecho GRASP MRI for Free-Breathing Fat/Water-Separated 3D T1 Mapping1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 3MR Application Development, Siemens Healthcare GmbH, Erlangen, Germany, 4Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States
Synopsis
This study aims to develop a framework called MP-Dixon-GRASP (Magnetization-Prepared Dixon-based Golden-angle RAdial Sparse Parallel MRI) for rapid free-breathing fat/water-separated 3D T1 mapping of the liver. The technique combines inversion recovery-prepared multiecho stack-of-stars acquisition with subspace-based sparse image reconstruction. The performance of MP-Dixon-GRASP was evaluated in fat/water phantoms and in subjects with normal and elevated liver fat content. The results suggested that fat/water-separated T1 mapping is able to remove the influence of fat, which enables more accurate estimation of true T1 values in the liver. With fat/water-separated T1 estimation, MP-Dixon-GRASP could be potentially useful for imaging patients with fatty-liver diseases.
Introduction
T1 mapping is a promising technique for evaluating different liver diseases, such as hepatic inflammation and fibrosis [1]. However, the presence of fat in the liver often presents a confounding factor for estimating the true T1 values of underlying liver parenchyma. Fat is known to have short T1, while conversely, inflammation/fibrosis tends to prolong T1 [2-3]. As such, standard T1 measurement in isolation can result in substantial bias, particularly when concomitant fat content in the liver is increased. Meanwhile, 3D T1 mapping of the liver remains a challenging task due to respiratory motion and the slow imaging speed of MRI. These limitations are all potential sources of poor reliability, accuracy and reproducibility in liver T1 measurement. The main purpose of this work is to develop a new technique called MP-Dixon-GRASP (Magnetization-Prepared Dixon-based Golden-angle RAdial Sparse Parallel MRI) for rapid free-breathing fat/water-separated 3D T1 mapping of the liver. By removing the influence of fat, more accurate T1 can be estimated to serve as an improved marker of liver inflammation/fibrosis.Methods
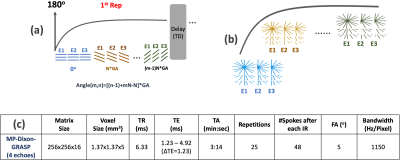
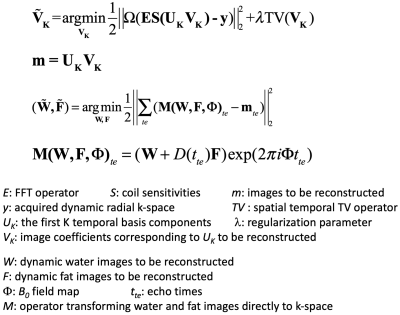
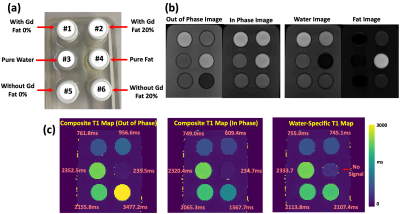
(a) MP-Dixon-GRASP MRI: MP-Dixon-GRASP acquisition combines a multiecho stack-of-stars sequence [4] with inversion recovery (IR) preparation. As shown in Figure 1a, multiecho radial stacks are continuously acquired after each IR preparation. This acquisition is repeated for multiple times to form a composite image series for T1 estimation, as shown in Figure 1b. MP-Dixon-GRASP reconstruction is shown Figure 2. Specifically, a subspace low rank and sparsity-based reconstruction algorithm, adapted from a previous technique (GRASP-Pro [5]), is implemented to reconstruct multiecho images at different inversion times (TIs), from which model-based fat/water separation is then performed to generate IR-prepared water and fat images. The water images are then used to fit water-specific (fat-corrected) T1 values.(b) Fat/Water Phantom Imaging: A home-made fat/water phantom was used to evaluate the performance of fat/water-separated T1 mapping using MP-Dixon-GRASP. The phantom contains 6 vials, including one with pure water, one with pure peanut oil, two with gadolinium (0.07936 mol/L MultiHance) but different fat fractions (0% and 20%), and two without gadolinium and with different fat fractions (0% and 20%), as shown in Figure 3a. Imaging was performed using a protocol shown in Figure 1c. ROI-based mean T1 values from the water-specific T1 map, the out-of-phase composite T1 map (from the first echo, TE=1.23ms), and the in-phase composite T1 map (from the second echo, TE=2.46ms) were compared for each vial.
(c) In-Vivo Imaging: MP-Dixon-GRASP was performed in 6 volunteers during free breathing using the same protocol. One subject was later confirmed to have liver steatosis. A total of 10 ROIs in the liver parenchyma were selected on different slices to compare water-specific T1 with out-of-phase and in-phase composite T1. Bland-Altman analysis and two-tailed paired student t test were performed to assess their differences.
Results
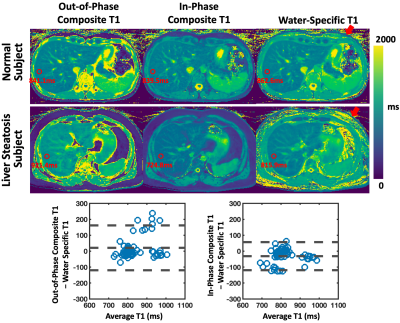
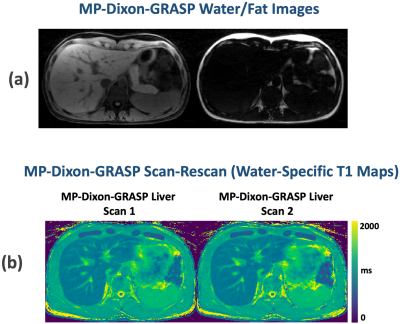
Figure 3b compares T1-weighted out-of-phase and in-phase phantom images together with separated water and fat images. Figure 3c compares the composite T1 maps with the water-specific T1 map from the phantom. When fat and water are not mixed, vials 1, vial 3, vial 4, vial 5 present similar T1 values across different T1 maps. When fat and water are mixed (vial 2 and vial 6), larger out-of-phase composite T1 and smaller in-phase composite T1 were obtained compared to corresponding vials without fat (vial 1 and vial 5, respectively). The water-specific T1 map indicates that the influence of fat can be removed, and similar underlying T1 values for [vial 1, vial 2] and [vial 5, vial 6] can be obtained despite different fat fractions. The T1 values from Figure 3c confirm the image contrast presented in corresponding T1-weighted images (Figure 3b).Figure 4 compares composite T1 maps with water-specific T1 maps in two subjects with Bland-Altman plots. The mean out-of-phase composite T1, in-phase composite T1, and water-specific T1 values of the liver are 854.31±90.65ms, 801.94±71.46ms, and 833.29±67.86ms, respectively. The water-specific T1 is significantly higher than the in-phase composite T1 (P<0.001) and is significantly lower than the out-of-phase composite T1 (P<0.05) in the liver. This finding is consistent with the results in the fat/water phantom experiment. Figure 5 shows representative fat/water-separated images and scan-rescan test of MP-Dixon-GRASP for water-specific T1 mapping in one subject.
Discussion
This work demonstrated the performance of MP-Dixon-GRASP, a new imaging technique for fat/water-separated T1 mapping. Compared to existing multiecho T1 mapping techniques that are mostly implemented in 2D with breath holds [6-11], our technique enables 3D free-breathing liver imaging. MP-Dixon-GRASP was first validated in fat/water phantoms, and we validated the hypothesis that water-specific T1 is independent of fat fractions. MP-Dixon-GRASP was then evaluated in 6 volunteers for liver T1 mapping, and we investigated the difference between water-specific T1 and out-of-phase and in-phase composite T1. We also tested the scan-rescan repeatability of MP-Dixon-GRASP. This initial study suggested that water-specific T1 mapping is able to remove the influence of fat, generating consistent underlying T1 values despite different fat fractions. The new technique could be potentially useful for imaging organs with elevated fat content, such as fatty livers.Acknowledgements
The authors would like to thank Renata Pyzik for help with the volunteer recruitments, and Kamil Banibaker and Dewey Chu for help with the MRI scans.References
1. Henninger, B. et al. Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. Eur. Radiol. 22, 2478–2486 (2012).
2. Mozes, F. E. et al. Influence of fat on liver T1 measurements using modified Look–Locker inversion recovery (MOLLI) methods at 3T. J. Magn. Reson. Imaging 44, 105–111 (2016).
3. Hoad, C. L. et al. A study of T1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 28, 706–714 (2015).
4. Benkert, T. et al. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn. Reson. Med. 78, 565–576 (2017).
5. Feng, L. et al. GRASP-Pro: imProving GRASP DCE-MRI through self-calibrating subspace-modeling and contrast phase automation. Magn. Reson. Med. 83, 94–108 (2020).
6. Jaubert, O. et al. Water–fat Dixon cardiac magnetic resonance fingerprinting. Magn. Reson. Med. 83, 2107–2123 (2020).
7. Jaubert, O. et al. Multi‐parametric liver tissue characterization using MR fingerprinting: Simultaneous T 1 , T 2 , T 2 *, and fat fraction mapping. Magn. Reson. Med. 84, 2625–2635 (2020).
8. Nezafat, M. et al. Imaging sequence for joint myocardial T 1 mapping and fat/water separation. Magn. Reson. Med. 81, 486–494 (2019).
9. Thompson, R. B. et al. Simultaneous proton density fat-fraction and R2∗ imaging with water-specific T1 mapping (PROFIT1): application in liver. Magn. Reson. Med. (2020) doi:10.1002/mrm.28434.
10. Liu, Y. et al. Myocardial T 1 and T 2 quantification and water–fat separation using cardiac MR fingerprinting with rosette trajectories at 3T and 1.5T. Magn. Reson. Med. mrm.28404 (2020) doi:10.1002/mrm.28404.
11. Li Z, et al. Rapid multi-slice fat and water separated T1 and composite R2* mapping using a dual-echo radial inversion recovery SPGR pulse sequence. ISMRM 2020, p312.
Figures