0749
Freeing MRI from its Faraday cage with Interference Rejection
Hadrien Dyvorne1, Todd Rearick1, Michael Poole1, Carole Lazarus1, Pierre Weiss2, Laura Sacolick1, Jeremy Jordan1, Cedric Hugon1, William Mileski1, Gang Chen1, Rafael O'Halloran1, Chris McNulty1, Jonathan Lowthert1, Anne Nelson1, Aristito Lorenzo1, Nicholas Zwart1, Prantik Kundu1, Scott Martin1, Andrei Loutchouk1, E. Brian Welch1, Samantha By1, Bradley Cahn3, Matthew Yuen3, Mercy Mazurek3, Anjali Pranhat3, Matthew Rosen4, Kevin Sheth3, and Jonathan Rothberg1
1Hyperfine, Guilford, CT, United States, 2ITAV, CNRS, Toulouse, France, 3Neurology, Yale University School of Medicine, New Haven, CT, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
1Hyperfine, Guilford, CT, United States, 2ITAV, CNRS, Toulouse, France, 3Neurology, Yale University School of Medicine, New Haven, CT, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
We introduce methods and hardware for rejection of high levels of interference from MR data, allowing the acquisition of MR imaging scans outside the shielded room and with no disruption to patient care. Interference cancellation relies on deriving a transfer function between “primary” sensors that detect MR signal and interference, and “reference” sensors that detect interference only. Interference rejection up to 67 dB is demonstrated, along with imaging results from scans acquired at the patient bedside in a Neuro ICU.
Introduction
Electromagnetic interference (EMI) is a well-known source of artifacts in MR imaging, and can lead to non-diagnostic image quality. Most MR systems to-date rely on shielded rooms to filter out EMI, adding cost and complexity to scanner installation. Additionally, critical hospital electronic devices are often excluded from entering the MR suite. Here we report a method that performs interference cancellation using auxiliary EMI sensors [1]. We further demonstrate that MR imaging can be performed outside the shielded room, at the patient bedside and in the presence of standard hospital equipment.Methods
A 16-channel acquisition system was designed for a low field portable MRI operating at 2.7 MHz, with channels assigned as follows: 8 “primary” surface coils mutually coupled to the patient’s head; 8 “reference” sensors, among which 7 coils placed around the instrument and a sensor capacitively coupled to the patient. Primary sensors detect both MR signal and interference. The total signal at time $$$t_k$$$ in primary sensor $$$i$$$ is:$$b_{p,i}(t_k)=b_{p,i}^S(t_k)+b_{p,i}^I(t_k)+b_{p,i}^N(t_k)$$
Where subscript p stands for primary, superscripts S, I, N stand for NMR signal, interference signal, and sensor thermal noise respectively. Reference sensors, on the other hand, detect interference signal only:
$$b_{r,j}(t_k)=b_{r,j}^I(t_k)+b_{r,j}^N(t_k)$$
After Fourier decomposition, the primary sensor's interference term $$$b_{p,i}^I$$$ can be expressed as a sum of reference sensor terms $$$b_{r,j}^I$$$, for each readout frequency $$$\omega_k$$$:
$$\widehat{b}_{p,i}^I(\omega_k)=\sum_j\alpha_{i,k,j}\widehat{b}_{r,j}^I(\omega_k)$$
The coefficients $$$\alpha_{i,k,j}$$$ are derived through least squares minimization using calibration data, which can be data acquired in absence of MR signal or MR data with a k-space weighting applied to reduce MR signal contribution to fit. Additionally, coefficient calibration may be updated throughout the acquisition in order to account for fluctuations in EMI sources. After determining the $$$\alpha_{i,k,j}$$$, corrected data (superscript C) is derived as:
$$\widehat{b}_{p,i}^C(\omega_k)=\widehat{b}_{p,i}(\omega_k)-\sum_j\alpha_{i,k,j}\widehat{b}_{r,j}(\omega_k)$$
Experiments were performed on the 64 mT Hyperfine SwoopTM portable MR system (Hyperfine, Guilford, USA). The system was equipped with a partial RF shield (with an opening for patient loading) that provides 3-39 dB of EMI attenuation, depending on receive coil position. Proof of concept: a free induction decay (FID) signal was acquired (with RF excitation) after 100 interference acquisitions (without RF excitation). Signal was acquired in 3 scenarios, a) baseline environment interference, b) presence of a nearby MR scanner’s RF pulses and c) high amplitude frequency sweep interference. Interference rejection performance was estimated using the ratio of raw to corrected data spectral density. Imaging: exams were performed on 11 subjects in the neurointensive care unit (Yale New Haven Hospital, USA), at the patient bedside and with standard hospital electronics operating in the room. This prospective study was IRB approved and all subjects gave consent. Fluid attenuated inversion recovery (FLAIR) images were acquired using an axial 3D inversion recovery fast spin echo sequence with inversion time 1.4 s, repetition time 4 s, voxel size 1.6 x 1.6 x 5 mm3, field of view 220 x 180 x 180 mm3, acquisition bandwidth 64 kHz, echo spacing 5.5 ms, 80 echoes per repetition, scan time 9:47 min.
Results
Figure 2 shows that interference is removed for all 3 scenarios, as evidenced by the corrected noise baseline (blue trace in c, f, i) being preserved. The maximum interference rejection achieved was 67 dB (h-i). This limit is set by hardware design choices such as sensitivity, dynamic range and number and placement of reference sensors. For imaging scans acquired in a hospital environment, the following equipment was either in proximity or attached to patients: EKG, vitals monitor, infusion pulp, IV drip, oxygen cannula. Average interference rejection was 24.4 dB (standard deviation 5.8 dB, range 3.2 - 41.2 dB). Low or negligible residual interference was observed after correction (average noise power density -30.8 dBm, range -26.1 to -33.4 dBm). Figure 3 shows an example FLAIR dataset from a stroke patient, all interference removed except for a faint residual line.Discussion
The multichannel sensor hardware and spectral-based interference rejection method presented here performed well in rejecting the majority of interference, in an uncontrolled hospital environment. Some of the residual observed might be attributed to nonlinear response of the sensor hardware, especially for high amplitude interference. Future work should assess the usefulness of nonlinear models for improved interference rejection, as well as alternative rejection filter design [2-4].Conclusion
We have demonstrated that EMI can be rejected from MR data using a collection of reference sensors and a digital subtraction filter. Such advances allow MR imaging to be performed outside of a shielded room, at the bedside, with standard electronics equipment operating nearby. Performing MRI exams at the patient bedside may reduce the burden of transporting critical patients to a controlled MRI suite, and lead to faster diagnostic decisions using MR imaging [5].Acknowledgements
No acknowledgement found.References
[1] Rearick, T., Charvat, G. L., Rosen, M. S., Rothberg, J. M. Noise Suppression Methods and Apparatus. US Patent 9,625,543 (2017).[2] Ferrara, E. R. Jr., Widrow, B. Multichannel Adaptive Filtering for Signal Enhancement. IEEE Transactions on Acoustics, Speech and Signal Processing 29, 766-770 (1981).[3] Maas, A. L. et al. Recurrent Neural Networks for Noise Reduction in Robust ASR. in Proc. INTERSPEECH, (2012).[4] Briggs, F. H., Bell, J. F., Kesteven, M. J. Removing Radio Interference From Contaminated Astronomical Spectra Using an Independent Reference Signal and Closure Relations. The Astronomical Journal, 120, 3351-3361 (2000).[5] Sheth, K. N. et al. Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurol. Published online September 08 (2020).Figures
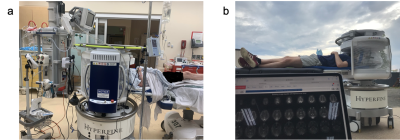
Example of portable scanner usage outside of a shielded room. Neurointensive Care Unit (a), with the following equipment surrounding the patient: IV drip, EKG, ancillary emergency equipment (vitals, oxygen/gas hook ups). Scanning a healthy volunteer outdoor (b) while operating the system with a power generator.
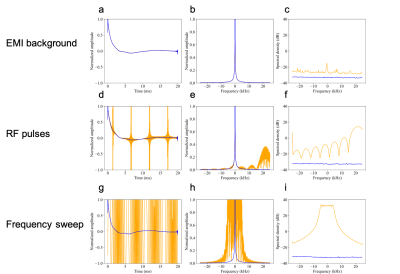
MR signal acquisition outside of a shielded room in background EMI (a-c), with RF pulses from a nearby scanner (d-e), and with a high amplitude frequency sweep (g-i). Orange trace: raw data, blue trace: corrected data. a, d, g: free induction decay, b, e, h: frequency spectrum, c, f, i: time-averaged noise power spectrum in absence of MR signal.
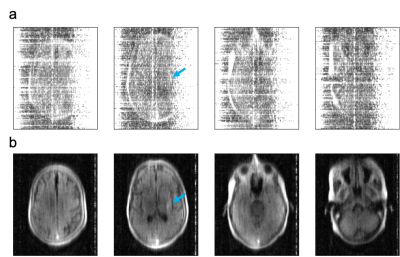
3D FLAIR images of a patient with a diagnosis of stroke, acquired at the bedside without RF shielded room. Images were reconstructed without (a) and with (b) the proposed interference rejection method. Average rejection was 29 dB (range 12-39 dB). Arrow points to pathology (distal M1 occlusion).