0747
Running Free on a Low-Field: a Proof of Principle1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5IHU LIRYC, Electrophysiology and Heart Modeling Institute, Fondation Bordeaux Université, Pessac-Bordeaux, France, 6Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Pessac, France, 7Magnetic Resonance, Siemens Healthcare, Erlangen, Germany
Synopsis
Low-field MR has recently attracted considerable attention because of reduced overall cost, lower field inhomogeneity, a lower specific absorption rate, and the potential for a more widespread global use. Owing to the simplicity and scalability of our recently developed free-running framework (FRF) for cardiovascular imaging, we here present the first results obtained with FRF at 0.55T. We demonstrate that FRF is scalable to this field strength by successfully reconstructing 5D cardiac- and respiratory motion-resolved whole heart images without the need of any gating or triggering devices, while reducing scan planning to a single mouse-click.
Introduction
For years, the MR research community has been pushing the boundaries of imaging capabilities for human applications by moving to ever-increasing magnetic field strengths. Even though 7T and beyond hold promise for extra signal-to-noise-ratio that can be spent to increase spatial resolution, acquisition speed, and diagnostic performance, it comes at substantially elevated costs and extra technical challenges. Simultaneously, it may be argued that higher and higher field strengths ultimately benefit fewer and fewer patients in clinical practice. Conversely, it logically follows that lower field systems may be less costly, that some of the technical challenges may be more easily addressed, and that more rather than fewer patients may be the ultimate beneficiaries. Paired with novel approaches for ease-of-use operation, lower initial purchasing investments, reduced siting costs, continuously improved computing power, as well as decreased maintenance and operating expenses, low-field scanners have tremendous potential of finding a more widespread use in low-to-middle income countries. Consequently, research and development in the domain of low-field MR has recently begun to gain momentum.1,2 While lower field strength comes at the expense of SNR, susceptibility is reduced which creates opportunity to run bSSFP sequences with a reduced bandwidth to compensate for the relative SNR penalty.3 Provided that SAR at lower field strength is reduced, bSSFP with a wider range of RF excitation angles may additionally be explored for endogenous contrast generation.1 For cardiovascular MR (CMR), bSSFP has become a mainstay and the recently-proposed free-running framework4 (FRF) enables free-breathing motion-resolved 3D imaging of the whole heart without the need of any gating or triggering devices, while simultaneously reducing scan planning to a single mouse click. Therefore, and at the confluence of modern low-field technology and easier approaches to imaging the heart, there seems to be an opportunity for making CMR more globally accessible. Here, we implemented FRF at 0.55T and tested the hypothesis that 3D images of the beating human heart can be acquired with a single mouse click without any gating or triggering devices.Methods
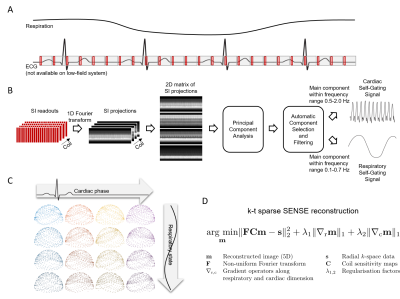
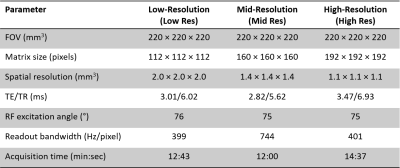
A version of a 3D radial free-running bSSFP sequence4 was implemented on a prototype 0.55T scanner (Siemens Healthcare, Erlangen, Germany) and tested in two healthy adult human subjects (Figure 1A). Three sets of data at three different spatial resolutions were acquired. Sequence parameters are shown in Figure 2. Raw data were reconstructed offline using a dedicated software.4 For total self-navigation in FRF, both respiratory and cardiac signals were directly extracted from the acquired free-running image data (Figure 1B). The resultant signal frequency spectra were plotted and visually validated. Using these physiological signals, data were then sorted into four respiratory bins and cardiac phases of 50ms width (Figure 1C) prior to k-t sparse SENSE image reconstruction (Figure 1D).4 This was followed by a high-dimensionality patch-based denoising, taking advantage of the large amount of spatial and temporal redundancies.5 Subsequently, overall image quality as well as resolution of both cardiac and respiratory motion were visually ascertained by expert readers.Results
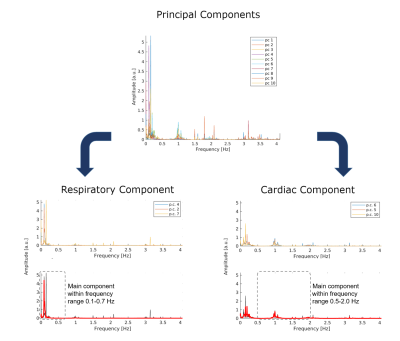
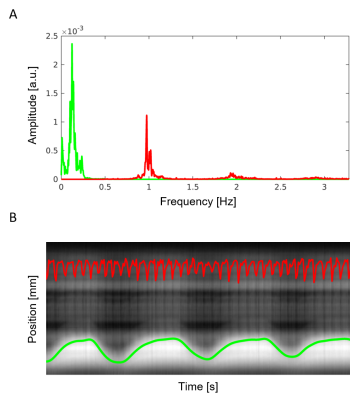
Self-gating signals were successfully extracted from all datasets. In Figure 3, the frequency spectrum obtained from the free-running data clearly displayed strong and separate respiratory and cardiac components. Visual inspection of these signals superimposed to the regularly acquired superior-inferior (SI) projections also validated the successful extraction of both self-gating signals (Figure 4). These signals were then used to sort the acquired data into 20 cardiac and 4 respiratory bins. Despite the absence of any gating and triggering, cardiac and respiratory motion were successfully resolved in all reconstructions (Figure 5). With an increase in spatial resolution, a concomitant decrease in SNR can be noticed as expected. Note that there is intrinsic contrast between the myocardium and the blood pool while fat signal appears bright.Discussion
These initial results obtained with FRF at 0.55T demonstrate that the framework is scalable and easily adaptable to low-field strength. The cardiac and respiratory signal components could easily be extracted from all datasets and physiological motion appears well-resolved in the final image reconstruction. Fat saturation was not yet applied and LIBRE6 or FISS7 may be among the future candidate fat suppression solutions. Simultaneously, the full range of parameters including RF excitation angles, repetition times, as well as bandwidth remain to be explored more mechanistically and systematically. In this initial study, the bandwidth for high and low spatial resolution remained unchanged yet this could easily be exploited for shortened scanning times. However, the effects on SNR and tissue contrasts also remains to be further elucidated. Owing to the simplicity and scalability of the FRF concept, 3D images of the beating human heart could successfully be acquired during free breathing on a 0.55T system with a single mouse click while no gating or triggering devices were needed. This simplicity, combined with all the advantages of low-field MRI, may help support dissemination CMR more globally.Conclusion
To our knowledge, this is the first time cardiac and respiratory motion-resolved free-running MRI has been reported on a 0.55T system. Self-gating signals could successfully be extracted from the acquired k-space data and be used for cardiac- and respiratory resolved 3D imaging of the human heart at that low-field strength.Acknowledgements
Davide Piccini and Jérôme Yerly contributed equally to this workReferences
1. Bandettini WP, Shanbhag SM, Mancini C, et al. A comparison of cine CMR imaging at 0.55 T and 1.5 T. Journal of Cardiovascular Magnetic Resonance 2020;22:37 doi: 10.1186/s12968-020-00618-y.
2. Sarracanie M, Salameh N. Low-Field MRI: How Low Can We Go? A Fresh View on an Old Debate. Front. Phys. 2020;8 doi: 10.3389/fphy.2020.00172.
3. Rashid S, Han F, Gao Y, et al. Cardiac balanced steady-state free precession MRI at 0.35 T: a comparison study with 1.5 T. Quantitative Imaging in Medicine and Surgery 2018;8:62736–62636.
4. Sopra LD, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magnetic Resonance in Medicine 2019;82:2118–2132 doi: 10.1002/mrm.27898.
5. Bustin A, Cruz GL da, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magnetic Resonance in Medicine 2019;81:3705–3719 doi: https://doi.org/10.1002/mrm.27694.
6. Masala N, Bastiaansen JAM, Sopra LD, et al. Free-running 5D coronary MR angiography at 1.5T using LIBRE water excitation pulses. Magnetic Resonance in Medicine 2020;84:1470–1485 doi: https://doi.org/10.1002/mrm.28221.
7. Bastiaansen JAM, Piccini D, Sopra LD, et al. Natively fat-suppressed 5D whole-heart MRI with a radial free-running fast-interrupted steady-state (FISS) sequence at 1.5T and 3T. Magnetic Resonance in Medicine 2020;83:45–55 doi: https://doi.org/10.1002/mrm.27942.
Figures