0745
Residential MRI: Fully Mobile Neuroimaging for Community and Population-Based Studies1Bill & Melinda Gates Foundation, Seattle, WA, United States, 2Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States, 3Pediatrics, Rhode Island Hospital, Providence, RI, United States, 4Hyperfine, Guildford, CT, United States, 5New England Collision, Seekonk, MA, United States
Synopsis
The continued increase in magnetic field strength and gradient capabilities of MRI systems has allowed ever more sophisticated interrogation of brain structure and function. However, these systems are often limited to high resource imaging research centers. To extend to more general population-studies, there is need for low-cost mobile imaging systems that allow for anywhere/everywhere imaging. In this work, we built a mobile MRI lab using the 64mT Hyperfine SwoopTM system and a customized Ford Transit cargo van, and demonstrated the ability to perform routine and rapid at-home MRI for the first time.
Introduction
MRI has enabled unprecedented advances in medical diagnosis and opened new opportunities for musculoskeletal, cardiovascular, and neuroscience research. However, a fundamental obstacle to its widespread adoption has been the significant infrastructure requirements. As MRI scanners have become more powerful (advancing main magnetic field strengths and increasing gradient performance), so too has the need for RF and magnetic shielding, uninterrupted power, and gradient cooling systems. Consequently, state-of-the-art systems are often limited to tertiary care settings and leading university research centers in predominately high income countries (HICs). This biases the individuals who participate in research studies - often skewing cohorts to higher socioeconomic demographics, limiting participation from rural communities, and challenging the extension of studies in many low and middle income countries (LMICs). To this end, the development of lower field MRI systems (<0.5T) may offer opportunities to move ‘beyond the lab’ into many of these under-represented communities(1). The Hyperfine SwoopTM (www.hyperfine.io), for example, has a permanent main magnetic field of 64mT, a 5 Gauss boundary diameter of ~5 feet, low power requirements, and is light (~1400lbs). The SwoopTM scanner was developed to increase access to MRI, but is currently only tested and FDA cleared for use at the point-of-care in US medical facilities, so we sought to evaluate the feasibility of home-based MRI, which could radically extend the reach of MRI clinical and research studies.Methods
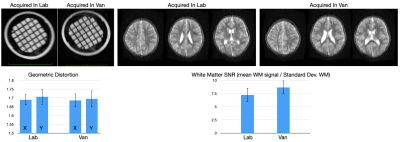
While current ‘portable’ MRI systems require 18-wheel haulers, high weight-capacity roadways, and have the same power needs of fixed systems, we sought a more flexible approach with 3 functional aims: 1. The ability to travel on local and dirt roads without a commercial license; 2. The ability to easily load and unload the scanner, for imaging in or outside the vehicle; 3. The ability to use either portable or fixed power. To achieve these aims, we customized a Ford Transit 2500 cargo van with a 9500lbs gross vehicle weight rating (Fig. 1a). An aluminum pallet was designed to allow easy scanner loading/unloading using a forklift or self-loading lifter (Fig. 1b). To address safety concerns, an integrated frame roll-cage was built that provided docking for the loading pallet and scanner and ensured the scanner would not tip or topple in case of cornering or de/acceleration (Fig. 1c). A portable RF shielded lithium-ion battery pack was also developed to provide portable power where necessary (Fig. 1d), in addition to a gas generator. This combination allows the system to be driven nearly anywhere, with minimal set up time.In demonstration, home-based MRI was performed across a series of individuals from 7 to 40 years of age at their residence, as well as reference scans collected from the same system at our research lab. These included geometric phantom and in vivo whole-brain T2-weighted fast spin echo anatomical scans, collected with the following parameters: TE/TR = 209/2000ms; receiver bandwidth = 64 kHz, echo train length = 80; isotropic resolution = 1.5 x 1.5 x 5mm; and acquisition time of just under 6mins. Data were visually inspected for off-resonance and main field inhomogeneity artifacts, and mean length/width of the geometric phantom elements were calculated and compared. Signal-to-noise measures were also calculated and compared between the mobile and stationary ‘in-lab’ scans.
Results & Discussion
A demonstration of at-home MRI is provided at https://www.youtube.com/watch?v=JRfmFpXQnRQ Despite the added weight, the van was able to travel at normal road and highway speeds, corner, and stop securely. Representative geometric phantom and in vivo T2-weighted images collected in the van and at the research center are shown in Fig. 2. Comparing mean length and width measures across the geometric phantom revealed no significant biases or field inhomogeneity differences; and white matter SNR measures were found to be consistent and comparable between the two settings. Thus, we found no significant image quality degradation or obvious visual artifacts were observed between the van or static scans.While portable EEG and NIRS systems have been previously used as part of mobile research labs(2), or used in rural or LMIC settings(3), this is the first demonstration of remote ‘point of science’ anywhere/everywhere imaging with MRI. The ability to take the scanner to the participant, coupled with every increasing availability of remote neurocognitive and biosample collections, breaks the current lab-centric focus of neuroscience research, and may help reduce the current race, gender, and socioeconomic biases in neuroscience research(4-6).
Acknowledgements
No acknowledgement found.References
1. Marques JP, Simonis FFJ, Webb AG. Low-field MRI: An MR physics perspective. J Magn Reson Imaging. Jun 2019;49(6):1528-1542. doi:10.1002/jmri.26637
2. Lau-Zhu A, Lau MPH, McLoughlin G. Mobile EEG in research on neurodevelopmental disorders: Opportunities and challenges. Dev Cogn Neurosci. Apr 2019;36:100635. doi:10.1016/j.dcn.2019.100635
3. Lloyd-Fox S, Papademetriou M, Darboe MK, et al. Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Sci Rep. Apr 22 2014;4:4740. doi:10.1038/srep04740
4. Shansky RM, Woolley CS. Considering Sex as a Biological Variable Will Be Valuable for Neuroscience Research. J Neurosci. Nov 23 2016;36(47):11817-11822. doi:10.1523/JNEUROSCI.1390-16.2016
5. Farah MJ. The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron. Sep 27 2017;96(1):56-71. doi:10.1016/j.neuron.2017.08.034
6. Abiodun SJ. "Seeing Color," A Discussion of the Implications and Applications of Race in the Field of Neuroscience. Front Hum Neurosci. 2019;13:280. doi:10.3389/fnhum.2019.00280
Figures