0742
Feasibility of accelerated diffusion weighted imaging for prostate cancer screening on prototype 0.55T system enabled with random matrix theory1Radiology, NYU School of Medicine, New York, NY, United States, 2Siemens Medical Solutions, New York, NY, United States
Synopsis
We compared standard and RMT reconstructions for a prostate DWI exam on a prototype 0.55T for varying number of averages (1 to 40) to evaluate the image quality and overall loss. For standard reconstructions, regardless of number of averages, the Rician bias is present even at 40 averages for high-b value data, whereas 1 average on RMT is sufficient to produce diagnostic grade DWI at 0.55T.
Introduction
PCa is the most common malignancy and the second leading cause of cancer-related mortality in men in the US. The American Cancer Society estimated >170,000 men were diagnosed with PCa and >32,000 men died in 2019 from this dreaded disease. A recent NIH report estimated the cost of PCa care in the US in 2018 to be >$15 billion. Despite such high incidence and curative treatment there is a lack of good screening test. Prostate Specific Antigen (PSA) has high sensitivity but lacks specificity for clinically significant PCa. Multi-parametric prostate MRI has become widely used in the detection and localization of clinically significant PC. Recently it was asserted that a low-cost/low-field, 0.55T system1, with modern hardware, may offer an interesting option for low-cost more accessible screening for various diseases such as PCa. Another advantage of low-field system is minimization of b0-distortion, which could obscure detection of PCa2. One major disadvantage of the low-field system is low signal to noise ratio (SNR) and corresponding long acquisition time. In order to accelerate DWI on low-field system we demonstrated the feasibility of accelerated diffusion weighted imaging on a prototype 0.55T system, by overcoming SNR limitations using the random matrix theory (RMT) reconstruction3,4.Methods
Acquisition. 5 volunteers were imaged with a 6-channel pelvic surface array coil and an 18-channel spine array (of which 6 and 2 coils were enabled, respectively) on a 45mT/m and 200 T/m/s commercial MRI system (1.5T MAGNETOM Aera Siemens Healthcare) modified to operate as a prototype system at 0.55T. DWI were acquired with 2 b=0, with 3-non-collinear directions of b=50 and b=1000, with 11 and 40 averages, respectively. Other imaging parameters include, TR = 4400 msec, TE=83 msec, bandwidth=1395 Hz/pix, voxel size=1.88x1.88x5, partial Fourier=6/8.Reconstruction. We employ the RMT reconstruction procedure3,4 to decorrelate coil noise5, demodulate the phase3,4 locally compress coil data into a virtual coil basis (MP-VCC)6; perform in line-averaging in the phase-less virtual coil basis; discard noise eigenvalues from a non-local spatial patch defined by the Marchenko-Pastur (MP) distribution7 determined from singular value decomposition of the local data matrix of $$$[N_{voxels}\cdot N_{VC}]\times[N_{Measurements}]$$$ around each voxel. For both standard and RMT reconstructions, partial Fourier is resolved with POCS and coil images are combined adaptively8.
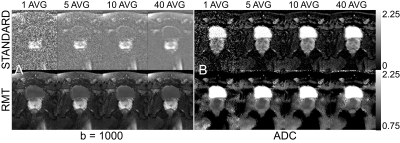
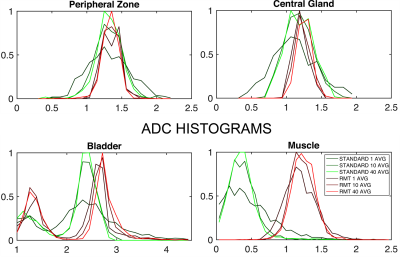
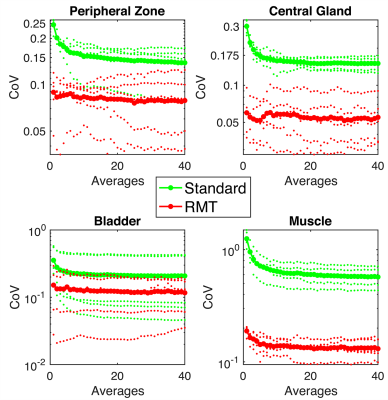
Comparison. 40 separate reconstructions were performed to include 1 to 40 b=1000 averages. We evaluate the precision and accuracy on ADC on standard and RMT reconstructions(with high averages as a bronze standard), using histogram analysis Fig.2 and change of coefficient of variation (COV=standard deviation/mean over an ROI) as a function of averages, Fig.3.
Results & Discussion
While precision of the standard reconstruction improves with the number of averages, at this low-field strength, b=1000 is artificially elevated due to proximity to the Rician noise floor(SNR<5). Therefore, ADC values in the standard reconstruction are lower than they appear on 1.5 or 3.0T. For all ROIs, ADC precision and values increase, indicating resolution of Rician bias. There is a substantial reduction in the noise floor of the standard reconstruction following RMT, Fig.1, which is apparent for all averages of b=1000. This effect is further illustrated, in the bladder ROI, which shifts towards 3.0, consistent with water at body temperature. For standard and RMT reconstructions, there is a steady decrease in CoV, that assymptotically converges either at a Rician noise floor or for biological variability, which appears to be the case for RMT, Fig.3. For peripheral zone and central gland regions, the CoV for standard reconstructions converge after 10 averages, while the RMT converges immediately (with 1-average). This indicates that the prostate protocol at 0.55T enabled by RMT may be as short or shorter than the clinical gold standard at 3.0T. For high SNR regions such as the peripheral zone, the CoV for some of the volunteers increases with the number of averages. This indicates that variability due to noise has been resolved and motion/mismatch between DWI may contribute to the ADC variability.Conclusion and Outlook
This work demonstrates the feasibility of performing DWI for multiparametric prostate screening at 0.55T. Using RMT to offset the SNR loss from the low-field strength, may yield an accelerated diagnostic grade clinical protocol that is insensitive to EPI-distortions.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing research agreement between NYU and Siemens Healthcare. This work was supported by the NIH under awards number R01NS088040 (NINDS) and R01EB027075 (NIBIB), and by the Center of Advanced Imaging Innovation and Research (CAI$$$^2$$$R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center: P41 EB017183.
References
1. Campbell-Washburn et al. “Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength mri,” Radiology293, 384–393 (2019), pMID: 31573398
2. Tong et al. “Exploratory study of geometric distortion correction of prostate diffusion-weighted imaging using b0 map acquisition,” Journal of Magnetic Resonance Imaging50, 1614–16195(2019)
3. Lemberskiy et al. “Achieving sub-mm clinical diffusion mri resolution by removing noise during reconstruction using random matrix theory,” In Proceedings 27nd Scientific Meeting 0770, International Society for Magnetic Resonance in Medicine, Montreal, Canada, 2019 (2019).
4. Lemberskiy et al. “Mri below the noise floor,” In Proceedings 28nd Scientific Meeting 0770, International Society or Magnetic Resonance in Medicine, Melbourne, Australia, 2020 (2020).
5. Roemer et al. “The NMR phased array,” Magnetic Resonance in Medicine16, 192–225 (1990).
6. Lemberskiy et al. “Marchenko-Pastur Virtual Coil Compression (MP-VCC),” Submitted, International Society for Magnetic Resonance in Medicine, Vancouver, Canada, 2021 (2021).
7. Veraart et al. “Denoising of diffusion mri using random matrix theory,” NeuroImage142, 394 – 406 (2016).
8. Walsh et al. “Adaptive reconstruction of phased arrayMR imagery,” Magnetic Resonance in Medicine43, 682–690 (2000)
Figures