0720
CEST Imaging of Nose-to-Brain Drug Delivery using Iohexol liposomes at 3T1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3City University of Hong Kong Shenzhen Research Institute, Shenzhen, China
Synopsis
Imaging-guided nose-to-brain drug delivery provide a non-invasive monitoring of drug delivery to brain, which increases effective dose via bypassing Blood-Brain-Barrier(BBB). Here, we investigated imaging of nanomedicine delivery via intranasal-administration using CEST-detectable mucus-penetrating-liposome(with 10%PEG). Liposomes were loaded with Iohexol(Ioh-Lipo) and CEST properties were examined both in-vitro and in-vivo by injecting into mouse nostril. Ioh-Lipo generated CEST contrast of 33.4% at 4.3 ppm in-vitro. which was also observed in nostril, olfactory-bulb and frontal-lobe after intranasal-administration at 3T. We demonstrated the liposomes detectability both in nostril and olfactory-bulb by CEST. The result demonstrates an approach for imaging-guided Nose-to-Brain Intranasal Liposomal Drug Delivery.
Introduction
Regarding drug delivery to the brain, intranasal administration method is more efficient than other administration methods such as oral or intravenous injection due to the presence of Blood-Brain-Barrier(BBB) which limits the ability of the administration of pharmacological agents to the CNS1,2. Intranasal injection delivers drugs through olfactory-bulb and olfactory-neuron directly into the brain bypassing the BBB. Currently, there is lack of non-invasive methods for monitoring the drug delivery and drug distribution in the brain after administration in-vivo. Chemical exchange saturation transfer(CEST) could be used to monitor liposome-based nanomedicine, its biodistributions and potentially the drug distribution3,4. Previous study has showed that ≥7mol%-PEG liposomes provide improved distribution and mucus penetrating property compared to 0 and 3 mol%-PEG liposomes via monitoring CEST contrast generated by BA5. Another study has showed CT contrast agent can be considered as a CEST contrast agent because of the unique amide protons that exchange relatively slow with the bulk water protons6,7,8. Herein, we developed a mucus-penetrating-liposome for intranasal drug delivery, which is with CEST detectable to facilitate the monitoring of drug distribution in the brain. The CEST contrast of Iohexol loaded liposome(Ioh-Lipo) at 4.3 ppm could be monitored both in phantom, and in nostril, olfactory bulb and frontal lobe after intranasal administration at 3T, showing the potential for image-guided Nose-to-Brain Intranasal liposomal drug delivery.Methods
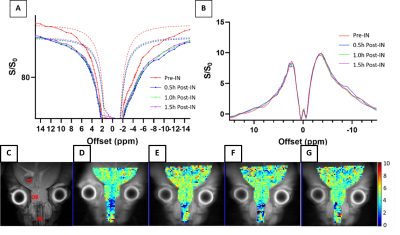
Ioh-lipo was prepared through thin film hydration method9 with a formulation of DSPC:cholesterol:DSPE-PEG2000=50:40:10 in chloroform with total weight of lipids of 50mg. Lipid mixture was evaporated to form a dry homogeneous thin film layer and then rehydrated with 1ml Iohexol solution. The suspension was annealed and extruded. The unloaded drug of Ioh-lipo was filtered through Sephadex G-50 gel columns twice before intranasal injection or imaging. Two groups of five 12-weeks-old ICR mice per group are used for intranasal administration10 of two mol% Ioh-Lipo. 200ul of Ioh-lipo was administrate intranasally by pipette into right nostril of ICR mouse. Treated mouse was hold for 30 mins for liposomes penetrating through the mucus and transported into the olfactory bulb. In-vitro MRI images for phantom experiments of Iohexol solutions and Ioh-lipo were acquired on horizontal bore 3T Bruker BioSpec system equipped with a 40-mm volume transceiver coil using a modified rapid acquisition with relaxation enhancement(RARE) sequence (Slice thickness=2 mm, field of view(FOV) =20x20 mm, image size = 32x32, RARE factor = 32, repetition time/echo time(TR/TE) = 6000/78.99 ms, -20 to + 20 ppm, 0.2 ppm steps with an extra acquisition point on ±4.1&4.3ppm). The in-vivo MRI images for intranasal administration experiments at the olfactory bulb of ICR mice were acquired pre-injection, 30mins, 1Hour, 1.5Hour after Intranasal injection on a horizontal bore 3T Bruker BioSpec system equipped with a 40-mm volume transceiver coil using a modified rapid acquisition with relaxation enhancement(RARE) sequence (Slice thickness=1.5 mm, field of view(FOV) =16x16 mm, image size = 64x64, RARE factor = 18, repetition time/echo time(TR/TE) = 5000/5 ms, -20 to + 20 ppm, 0.2 ppm steps with an extra acquisition point on ±4.1&4.3ppm). The T2 image in coronal view and CEST Z-spectrum were acquired and CEST contrast was calculated by applying Lorentzian fitting on Z spectra(CEST contrast%) for analysis of penetrating efficacy by intranasal administration and drug distribution in the olfactory bulb in treated ICR mouse.Results and Discussion
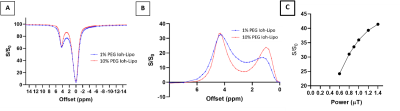
We prepared Ioh-Lipo with two different mol% of PEG, i.e. at 1% and 10%. As shown in Fig.1, both formulations of Ioh-Lipo showed CEST contrast of 33.5% at 4.3 ppm at the optimized power of 0.9 μT in-vitro. The particle size, polydispersity index and zeta potential were 166.3 nm, PDI=0.175, -4.84 mV for 10% PEG Ioh-Lipo and 173.9nm, PDI=0.170, -3.74mV for 1% PEG Ioh-Lipo, and the particle concentration was 9.27±2.46 e+7 particles/ml and 3.67±1.12e+7 particles/ml respectively. As shown in Fig. 2&3, the 1mol% and 10mol% Ioh-Lipo was injected intranasally into right nostril of ICR mouse. The in-vivo MRI T2 image in coronal view, CEST Z-spectrum, CESTLDFit and CEST-map at the olfactory bulb of treated ICR mice were acquired pre-injection, 0.5hr, 1hr, 1.5hr after intranasal injection. Mice received 1%mol PEG Ioh-Lipo showed a CEST contrast of 4.47% and 4.60% at 4.3ppm pre-injection and 1.5hr post-injection respectively, while mice received 10%mol PEG Ioh-Lipo showed a CEST contrast of 4.72% and 6.41% at 4.3ppm pre-injection and 1.5hr post-injection respectively at the region of olfactory bulb accumulated with time. Notably, Ioh-Lipo was only injected into right nostril, the liposome can reach the olfactory bulb and the frontal lobe successfully with slightly higher CEST contrast at the injected side. The increase in CEST contrast at 4.3 pmm is higher for 10%mol PEG Ioh-Lipo than in 1mol% PEG Ioh-Lipo in-vivo, which could indicate the benefit of mucus penetrating property of liposomes with high mol%-PEG. This is important to ensure high efficiency of nose-to-brain drug delivery. This unique contrast property and mucus penetrating property of Ioh-Lipo could facilitate image-guided nose-to-brain drug delivery and further applications on image-guided treatment are underway.Conclusion
Here, we developed a brain-administrable mucus-penetrating intranasal liposome. Our Ioh-Lipo have CEST contrast at 4.3 ppm in-vitro indicates the Iohexol encapsulation. By monitoring this unique CEST contrast, tracking and monitoring of Ioh-Lipo distribution inside the brain and drug delivery from nose-to-brain can be achieved.Acknowledgements
This study was supported by Research Grants Council: 11102218; City University of Hong Kong: 7005210, 9680247, 9667198 and 6000660; National Natural Science Foundation of China: 81871409.References
1. Crowe, T. P., Greenlee, M. H., Kanthasamy, A. G., & Hsu, W. H. (2018). Mechanism of intranasal drug delivery directly to the brain. Life Sciences,195, 44-52. doi:10.1016/j.lfs.2017.12.025
2. Sun, B.-L., Wang, L.-H., Yang, T., Sun, J.-Y., Mao, L.-L., Yang, M.-F., … Yang, X.-Y. (2018). Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Progress in Neurobiology, 163-164, 118–143. doi: 10.1016/j.pneurobio.2017.08.007
3. van Zijl, P. C., & Yadav, N. N. (2011). Chemical exchange saturation transfer (CEST): what is in a name and what isn't?. Magnetic resonance in medicine, 65(4), 927–948. https://doi.org/10.1002/mrm.22761
4. Zhang, X‐Y, Wang, F, Li, H, et al. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR in Biomedicine. 2017; 30:e3716.
5. Yu, T., Chan, K. W., Anonuevo, A., Song, X., Schuster, B. S., Chattopadhyay, S., . . . Hanes, J. (2015). Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: Demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomedicine: Nanotechnology, Biology and Medicine,11(2), 401-405. doi:10.1016/j.nano.2014.09.019
6. Aime, S., Calabi, L., Biondi, L., De Miranda, M., Ghelli, S., Paleari, L., Rebaudengo, C. and Terreno, E. (2005), Iopamidol: Exploring the potential use of a well‐established x‐ray contrast agent for MRI. Magn. Reson. Med., 53: 830-834. https://doi.org/10.1002/mrm.20441
7. Chan KW, Yu T, Qiao Y, et al. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2014 Apr;180:51-59. DOI: 10.1016/j.jconrel.2014.02.005.
8. Hanson, L. R., Fine, J. M., Svitak, A. L., & Faltesek, K. A. (2013). Intranasal administration of CNS therapeutics to awake mice. Journal of visualized experiments : JoVE, (74), 4440. https://doi.org/10.3791/4440
Figures