0717
Improved volumetric inhomogeneous magnetization transfer (ihMT) using a CSF-suppressed FSE sequence (FLAIR-ihMT)1Division of MRI research, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2CRMBM, Aix-Marseille Univ, CNRS, Marseille, France
Synopsis
3D myelin imaging using inhomogeneous magnetization transfer (ihMT) has seen increased interest as sequence implementations evolve. This has mostly been based on prepared or pseudo-steady-state gradient echo sequences. However, Fast-Spin-Echo (FSE) sequences should have a theoretical SNR advantage over GRE. Here we report implementation of a CSF-suppressed FSE sequence for ihMT imaging (FLAIR-ihMT), exploring through simulations the effect of CSF suppression and providing first imaging results, highlighting the benefits of CSF suppression, potential for whole brain high-resolution imaging and translation down to the spinal cord.
Introduction
3D myelin imaging using inhomogeneous magnetization transfer1 (ihMT) has seen increased traction as sequence implementations now provide good SNR in a reasonable acquisition time and whole brain coverage, based on either prepared2 or pseudo-steady-state3,4 gradient echo implementations. 3D-ihMT has been used now in a variety of neuroscience5,6 and clinical applications7. Although under-explored, volumetric fast-spin-echo8 acquisitions should provide a theoretical SNR advantage over GRE from using multiple refocusing pulses. While early experiments have shown these benefits9, significant issues arising from CSF and eye motion leading to phase ghosting have been problematic. Inversion-recovery based CSF suppression, akin to FLAIR imaging is a potential solution, however careful consideration of its effect on the ihMT signal and contrast is required. Here we report implementation of such a CSF-suppressed FSE sequence for ihMT imaging, exploring through simulations the effect of CSF suppression and providing first imaging results.Material and Methods
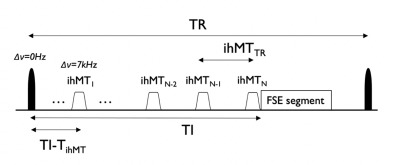
Sequence: We implemented FLAIR-ihMT built on a previous FSE sequence9 that included a non-selective inversion (Fig1) using a 15.36ms C-shaped FOCI10 (BW=10.8kHz,B1=14μT,β=809s-1,μ=3.9) pulse placed before the ihMT preparation at a time TI prior to imaging.Simulations: A model for the ihMT signal2,11 was modified to include the effect of instantaneous inversion of the free pool at TI-TihMT before the beginning of a TihMT long ihMT preparation. The resulting ihMTz was modelled as well as the ihMT signal expected following the first excitation pulse in both MPRAGE and IR-FSE. The TI was chosen to null CSF assuming T1,CSF=4s,TRFSE=6000ms, last echo sampling TElast=380ms (compatible with experiments) and following:
$$S(TI)=S_0\times(1-2\exp(-\frac{TI}{T_{1,CSF}}+\exp(-\frac{TR_{FSE}-TE_{LAST}}{T_{1,CSF}}))$$
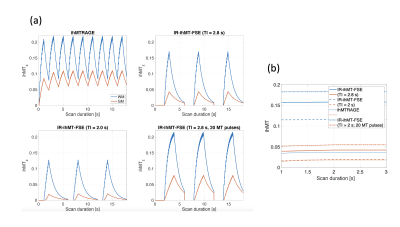
We preliminarily explored two TIs of 2s (providing total CSF suppression) and 2.8s (providing CSF attenuation to ≈10% but allowing more WM/GM Mz recovery prior to the ihMT preparations).
Experiments: N=4 volunteers were recruited and scanned on a 3T scanner (GE Discovery MR750) using a 32-ch head coil. In N=3 subjects, we acquired ihMT-RAGE data (TRMPRAGE=2s,90 views/segment, 2x2 ARC acceleration, flip-angle=10°,Tacq=2mins12s) as well as an IR-FSE (TI/TR/TE=2000/6000/8ms, ETL=100, 2x2 ARC acceleration, variable refocusing flip-angles, Tacq=5mins57s). All ihMT acquisitions used the same low RF duty-cycle preparation12 (ten 5-ms long Tukey pulses repeated every 100ms with a peak B1=14μT, frequency offset=7kHz) and both MPRAGE and IR-FSE were acquired with the same (2.4mm)3 resolution with a single-average. In each case, a zero-power M0 reference volume was acquired for computation of an ihMT ratio (ihMTR=(MT++MT--2*MTdual)/M0). For comparison across sequences, we calculated a normalized ihMT signal by dividing signal in two ROIs (thalamus and pons) by an ROI positioned in the posterior neck muscles from which ihMT is lower. In addition, in one volunteer, we acquired an ihMT-FSE without any preparatory inversion to confirm the benefits of CSF suppression. To demonstrate the potential SNR and image quality gain associated with the use of FLAIR-ihMT, we also acquired in this subject a 1.6mm isotropic high-resolution dataset using a variable-density k-space sampling in 15 minutes, reconstructed using an L1-wavelet Compressed-Sensing reconstruction13 followed by non-local means denoising assuming Rician noise distribution14. Finally, we scanned an additional volunteer using a 16-ch neurovascular coil to show potential spinal cord application. We acquired a T2-FSE (Tacq=1min,TR/TE=4000/96ms) as well as a flow-compensated FLAIR-ihMT (Tacq=7min,B1ihMT=12μT,1.8x1.8x2.4mm3).
Results
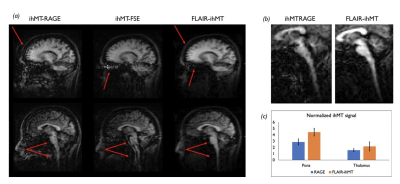
Simulations (Fig2) show that while the ihMTz at the end of the preparatory period is lower when using an IR-prepped FSE sequence compared to ihMT-RAGE, the actual ihMT SNR after the initial excitation flip-angle is expected to be up to 3x higher in WM at TI=2s using an IR-FSE because of the 90° excitation flip-angle compared to the typical 8-10° used in MPRAGE. A similar compensatory mechanism is expected in GM. Additional signal enhancement especially in the GM can be achieved by increasing the duration of the ihMT preparation without any TR penalty in the IR-FSE, albeit increasing SAR.Experiments confirm simulated results and highlight the further enhancement in image quality compared to both ihMT-RAGE and ihMT-FSE achieved when using FLAIR-ihMT as highlighted in Fig3a, with removal of ghosting artifacts originating from eyes and CSF motion. Also, one can appreciate the SNR and quality improvement in subtentorial regions as highlighted in Fig3b. Quantitatively, as seen in Fig3c, experiments are consistent with simulations with a higher normalized ihMT signal increase in the WM (+55%) compared to the GM (+37%).
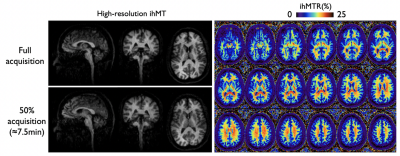
The higher SNR allowed acquisition of high-resolution ihMT volumes as seen in Fig4. Even with an additional 50% undersampling (leading to an R≈6 acceleration for a 7.5min scan), high-quality images were successfully reconstructed.
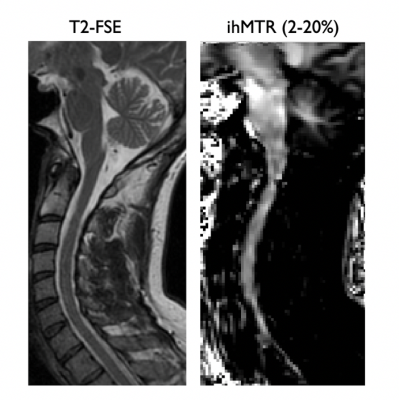
Finally, although extremely preliminary, the FLAIR-ihMT sequence can be successfully applied in the cervical spinal cord as highlighted in Fig5.
Discussion/Conclusions
Although currently slower than ihMT-RAGE for equal sampling and resolution, we have shown that an CSF-suppressed FSE sequence has tremendous potential for 3D-ihMT myelin imaging, outperforming GRE-based acquisitions in SNR and image quality. Combination with variable-density sampling paves the way for pushing the resolution limits of myelin-specific imaging of the central nervous system by providing scan time savings to be used for further signal improvement through use of longer ihMT preparation times or signal recovery to improve SNR. In addition, future spinal cord applications show great potential as CSF pulsatile motion is one of the main hurdles when translating brain developments down to the cord15–17.Acknowledgements
No acknowledgement found.References
1. Varma, G., Duhamel, G., Bazelaire, C. de & Alsop, D. C. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magnetic Resonance in Medicine 73, 614–622 (2015).
2. Varma, G. et al. Three-dimensional inhomogeneous magnetization transfer with rapid gradient-echo (3D ihMTRAGE) imaging. Magnetic Resonance in Medicine n/a,.
3. Wood, T. C. et al. Silent myelin-weighted magnetic resonance imaging. Wellcome Open Res 5, (2020).
4. Mchinda, S. et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magnetic Resonance in Medicine 79, 2607–2619 (2018).
5. Geeraert, B. L. et al. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. NeuroImage (2017) doi:10.1016/j.neuroimage.2017.09.019.
6. Munsch, F. et al. Characterization of the cortical myeloarchitecture with inhomogeneous magnetization transfer imaging (ihMT). NeuroImage 225, 117442 (2021).
7. Zhang, L. et al. A comparison study of inhomogeneous magnetization transfer (ihMT) and magnetization transfer (MT) in multiple sclerosis based on whole brain acquisition at 3.0 T. Magnetic Resonance Imaging 70, 43–49 (2020).
8. Busse, R. F., Hariharan, H., Vu, A. & Brittain, J. H. Fast spin echo sequences with very long echo trains: Design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magnetic Resonance in Medicine 55, 1030–1037 (2006).
9. Taso, M. et al. Variable-density Fast-Spin-Echo (FSE) for volumetric inhomogeneous Magnetization Transfer (ihMT) imaging. in Proceedings of the 28th annual meeting of the ISMRM 3140 (2020).
10. Ordidge, R. J., Wylezinska, M., Hugg, J. W., Butterworth, E. & Franconi, F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn. Reson. Med. 36, 562–566 (1996).
11. Varma, G. et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. Journal of magnetic resonance 260, 67–76 (2015).
12. Varma, G. et al. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. Journal of Magnetic Resonance 296, 60–71 (2018).
13. Uecker, M. et al. Berkeley Advanced Reconstruction Toolbox. in Proc. Intl. Soc. Mag. Reson. Med 2486 (2015).
14. Manjón, J. V., Coupé, P., Martí‐Bonmatí, L., Collins, D. L. & Robles, M. Adaptive non-local means denoising of MR images with spatially varying noise levels. Journal of Magnetic Resonance Imaging 31, 192–203 (2010).
15. Taso, M. et al. Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed 29, 817–832 (2016).
16. Girard, O. M. et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Improved imaging strategy for spinal cord applications. Magn Reson Med 77, 581–591 (2017).
17. Troalen, T. et al. Cervical Spine inhomogeneous Magnetization Transfer (ihMT) Imaging Using ECG-Triggered 3D Rapid Acquisition Gradient-Echo (ihMT-RAGE). in Proceedings of the 27th annual meeting of the ISMRM 300 (2019).
Figures