0713
Continuous 4D atlas of normal fetal lung development and automated CNN-based lung volumetry for motion-corrected fetal body MRI1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3Prenatal Cell and Gene Therapy, Elizabeth Garrett Anderson Institute of Women’s Health, University College London, London, United Kingdom, 4Stem Cells and Regenerative Medicine, GOS-UCL Institute of Child Health, London, United Kingdom, 5Department of Congenital Heart Disease, Evelina Children’s Hospital, London, United Kingdom, 6Department of Radiology, University Hospitals Leuven, Leuven, Belgium, 7Department of Imaging and Pathology, Biomedical Sciences, KU Leuven, Leuven, Belgium, 8Department of Obstetrics, University Hospitals KU Leuven, Leuven, Belgium, 9Paediatric Radiology, Evelina London Children’s Hospital, London, United Kingdom
Synopsis
This work presents a continuous 4D atlas of fetal lung development within 22-32 weeks gestational age (GA) generated from ~130 motion-corrected fetal body MRI datasets. The corresponding growth charts for fetal MRI lung indices are used for definition of the of normal ranges. In addition, we implemented and evaluated an automated method for fetal lung volumetry based on 3D UNet segmentations.
Introduction
Fetal MRI allows for detailed screening of fetal lung anomalies [1,2]. The ratio between the observed and the GA-specific expected [3,4] (O/E) total lung volume (TLV) values is routinely used in quantitative assessment of abnormal cases (e.g., congenital diaphragmatic hernia [5]). Calculation of TLV requires segmentation, which is normally performed manually in 2D on motion-corrupted MRI stacks with limited accuracy, which limits the validity required for wider clinical adoption.In an earlier reported T2w fetal body atlas with weekly templates [7] the image quality was severely compromised by blurring caused by nonrigid inter-slice motion. The recently proposed deformable slice-to-volume registration (DSVR) [8] resolves nonrigid motion and allows reconstruction of high-resolution 3D images of fetal body from multiple MRI stacks. DSVR images provide detailed information on the lung structure and intensity, which facilitates 3D segmentation[9].
This work proposes a continuous 4D atlas of fetal lung development within 22-32 weeks gestational age (GA) based on DSVR reconstructions of ~130 fetal MRI datasets combined using Gompertz model fitting. We further implemented an automated fetal lung volumetry method based on 3D UNet [10] segmentation.
Method
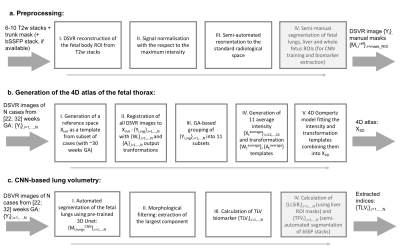
Datasets and preprocessing: This study includes iFIND [11] MRI datasets of 132 fetuses without reported anomalies scanned within 22-32 weeks GA range. The acquisitions were performed on a 1.5T Philips Ingenia system using T2w ssFSE: TR=15000ms, TE=80ms, voxel size 1.25x1.25mm, slice thickness 2.5mm and spacing 1.25mm. The bSSFP stacks (TR=5ms, TE=2.5ms, voxel size 1.5x1.5mm, slice thickness 2.5mm) were used for estimation of the total fetal volume (TFV) for 100 datasets. The preprocessing (Fig1.a.) included: (i) DSVR reconstruction [8] of the fetal trunk with 0.85mm resolution from 6-10 T2w stacks using SVRTK [12] ; (ii) intensity normalization; and (iii) reorientation to the standard radiological position using semi-automated landmark-based registration (MIRTK [13]). For network training 100 input lung segmentations were created by label propagation [13,14] followed by manual refinement (3DSlicer [15]).4D atlas: The proposed pipeline for generation of the 4D atlas is given in Fig.1.b. The initial reference space X_init was created by generation of an average template of from 16 DSVR images (~ 30 weeks GA). All images were then registered to X_init using affine + LNCC Demons in MRtrix3 [1]: {Y_i,reg},i=0,…,N. Next, all cases were grouped into 11 subsets (each with 10-18 cases) based on the GA and 11 discrete templates {X_t,},t=22,…,32 were generated using weighted averaging. The output transformations were averaged resulting in 11 non-linear warps {W_t} and affine parameters {A_t}. The fitting of the signal intensity, warps and affine transformations in 4D was performed based on the Gompertz model: G(t)=(β-𝛿)exp(-exp(-γ(τ-t))). Finally, the fitted 4D transformations were applied to the fitted 4D intensity image resulting in a continuous X_4D atlas with 0.5mm resolution.
Lung segmentation and biomarkers: Fig.1.c shows the proposed pipeline for extraction of the fetal lung biomarkers including: (i) automated segmentation of the lungs based on a classical 3D UNet implemented in Pytorch[17]; (ii) morphological filtering of the largest component; and (iii) computation of the average lung intensity values and the TLV. We used 55 cases for training with TorchIO [16] augmentation (linear transformations), 5 for validation and 40 for testing. All images were cropped to the thorax ROI and resampled with padding to 128x128x128 grid. The code is available at SVRTK repository.
In order to assess correlation of TLV with other biomarkers, we estimated the liver to lung signal intensity ratio (LLSiR) index [6] (the average liver intensity estimated from 3 small ROIs) and the total fetal volume (TFV) [1] values were extracted using 3D UNet-based segmentation of bSSPF stacks followed by manual refinement for 100 cases.
Results
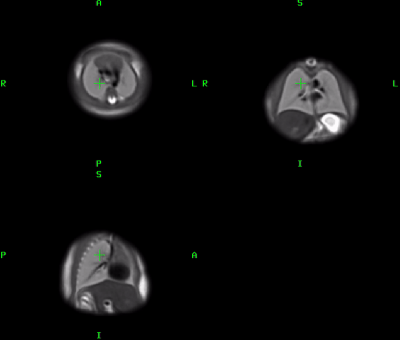
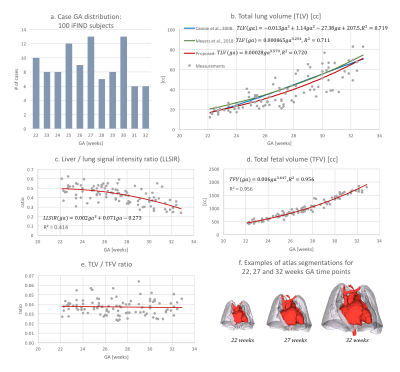
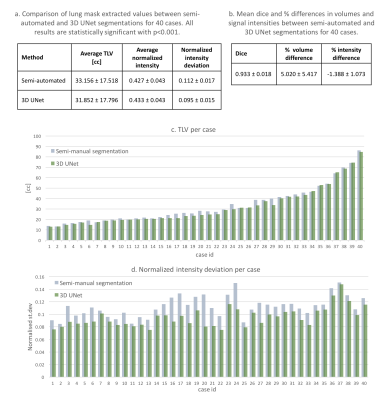
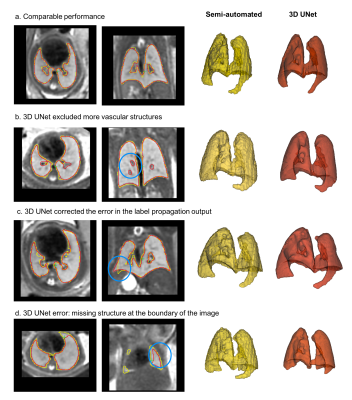
4D atlas and growth charts: The generated 4D atlas is presented in Fig.2. In addition to the changes in volume, there are also noticeable changes of lung intensity corresponding to maturation (reflected in the LLSiR chart Fig.3.b). The growth charts for 100 subjects (Fig.3) show relationship between TLV, TFV and LLSiR trends. There is a very strong correlation (R2>0.99) with the existing TLV formulas [3,4]. The atlas is available at SVRTK repository [12].Lung segmentation: The comparison between the semi-automated segmentations and the 3D UNet outputs was performed for 40 cases in terms of Dice, volume and intensity differences (Fig.4.a-d). There is a strong correlation between TLV values (p<0.001, average Dice ~0.933) with the ~5% mean difference attributed to the more conservative CNN segmentations. The 3D UNet removed inconsistencies (e.g., vessels, Fig.5.b) in comparison to the semi-automated segmentations thus resulting in smaller intensity deviations (Fig.4.d), higher mean lung intensity and slightly smaller TLV values. It also outperformed the label propagation in 17 of 40 cases based on qualitative evaluation (e.g., Fig.5.c). It partially failed only in two cases where the lung ROI was too close to the image border (Fig.5.d).
Conclusions
This work presented a high-resolution continuous 4D atlas of fetal lung development for 22-32 weeks GA along with the lung biomarker growth charts from 100 subjects. We also demonstrated that using 3D CNN-based segmentation of DSVR images allows fast and robust automated lung volumetry for fetal MRI in comparison to the conventional approach. Future work will focus on optimization of the automated organ segmentation for abnormal cases.Acknowledgements
We thank everyone who was involved in acquisition of the datasets and all participating mothers.
The iFIND project data used in this research were collected subject to the informed consent of the participants. This work was supported by the NIH Human Placenta Project grant[1U01HD087202-01], the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the WellcomeTrust and EPSRC IEH award [102431] for the iFIND project and by the National Institute for Health Research (NIHR) Biomedical ResearchCentre based at Guy’s and St Thomas’ NHS Foundation Trust and King’sCollege London.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
[1] L. Story, T. Zhang, J. Steinweg, J. Hutter, J. Matthew, T. Dassios, P. Seed, D. Pasupathy, J. Allsop, J. Hajnal, A. Greenough, A. Shennan, and M. Rutherford, “Foetal lung volumes in pregnant women who deliver very preterm: a pilot study,” Pediatr. Res., vol. 87, no. 6, pp. 1066–1071, 2020.
[2] E. Snyder, A. Baschat, T. A. G. M. Huisman, and A. Tekes, “Value of Fetal MRI in the Era of Fetal Therapy for Management of Abnormalities Involving the Chest, Abdomen, or Pelvis,” Am. J. Roentgenol., vol. 210, no. 5, pp. 998–1009, 2018.
[3] M. Cannie, J. Jani, F. Van Kerkhove, J. Meerschaert, F. De Keyzer, L. Lewi, J. Deprest, and S. Dymarkowski, “Fetal body volume at MR imaging to quantify total fetal lung volume: Normal ranges,” Radiology, vol. 247, no. 1, pp. 197–203, 2008.
[4] M. L. Meyers, J. R. Garcia, K. L. Blough, W. Zhang, C. I. Cassady, and A. R. Mehollin-Ray, “Fetal Lung Volumes by MRI: Normal Weekly Values From 18 Through 38 Weeks’ Gestation.,” Am. J. Roentgenol., vol. 211, no. 2, pp. 432–438, Aug. 2018.
[5] A. Debus, C. Hagelstein, A. Kilian, C. Weiss, S. Schonberg, T. Schaible, K. Neff, and K. Busing, “Fetal lung volume in congenital diaphragmatic hernia: Association of prenatal MR imaging findings with postnatal chronic lung disease,” Radiology, vol. 266, no. 3, pp. 887–895, 2013.
[6] M. Moshiri, L. Mannelli, M. L. Richardson, and P. Bhargava, “Fetal Lung Maturity Assessment With MRI Fetal Lung-to-Liver Signal-Intensity Ratio,” no. December, pp. 1386–1390, 2013.
[7] T. Zhang, M. Deprez, P. Aljabar, R. Wright, A. Davidson, M. Rutherford, J. Hajnal, and J. Schnabel. 2018. “A Spatiotemporal Fetal MRI Atlas for Multi-Organ Segmentation and Growth Analysis.” In ISMRM 2018, 654.
[8] A. Uus, T. Zhang, L. Jackson, T. Roberts, M. Rutherford, J. Hajnal, and M. Deprez, “Deformable Slice-to-Volume Registration for Motion Correction of Fetal Body and Placenta MRI,” IEEE Trans. Med. Imaging, vol. 39, no. 9, pp. 2750–2759, 2020.
[9] A. Uus, J. Davidson, J. Matthew, M. Deprez, and M. Rutherford, “Motion corrected fetal body MRI for 3D fetal lung volume assessment.” In BAPS 2020, 381, 2020.
[10] Çiçek, A. Abdulkadir, S. Lienkamp, T. Brox, and O. Ronneberger, “3D U-net: Learning dense volumetric segmentation from sparse annotation,” in MICCAI 2016, 10 2016, pp. 424–432.
[11] “iFIND Project.” [Online]. Available: http://www.ifindproject.com/. [Accessed: 01-Dec-2020].
[12] “SVRTK: MIRTK based SVR package for fetal MRI.” [Online]. Available: https://github.com/SVRTK/. [Accessed: 01-Dec-2020].
[13] “MIRTK package.” [Online]. Available: https://github.com/BioMedIA/MIRTK/. [Accessed: 01-Dec-2020].
[14] D. Rueckert, L. I. Sonoda, C. Hayes, D. L. G. Hill, M. O. Leach, and D. J. Hawkes, “Nonrigid Registration Using Free-Form Deformations: Application to Breast MR Images,” IEEE Trans. Med. Imaging, vol. 18, no. 8, pp. 712–721, 1999.
[15] “3DSlicer platform.” [Online]. Available: https://www.slicer.org/. [Accessed: 01-Dec-2020].
[16] “MRtrix3 toolbox.” [Online]. Available: https://www.mrtrix.org/. [Accessed: 01-Dec-2020].
[17] “PyTorch framework.” [Online]. Available: https://pytorch.org/. [Accessed: 01-Dec-2020].
[18] F. Pérez-García, R. Sparks, and S. Ourselin, “TorchIO: a Python library for efficient loading, preprocessing, augmentation and patch-based sampling of medical images in deep learning,” arXiv:2003.04696 [cs, eess,stat], Mar. 2020, arXiv: 2003.04696.
Figures