0710
Comparison of Pure Deep Learning Approaches for Placental Extraction from Dynamic Functional MRI sequences between 19 and 37 Gestational Weeks.1University of Washington, Seattle, WA, United States
Synopsis
We present fully automated deep learning approaches to placental tissue segmentation on our dataset of 68 3D R2* images. Using this dataset, we employ different data schemes to get 4 new datasets consisting of full 3D images, full 2D slices, 3D patches and 2D patches. An unmodified U-Net architecture is trained and tested on these datasets to evaluate the robustness of the model when presented with different data. We find that by artificially increasing the size of the dataset, the model is able to perform better at the segmentation task.
Introduction
Non-invasive functional imaging of the human placenta using quantitative MRI techniques1,4,5 over an extended time series provides a unique window to evaluate placental health. The first step in the automated analysis of such large scale 3D datasets is to accurately separate the placental anatomy from that of the mother and fetus. This is challenging because of the inherent variability in the shape and contrast of the organ and its surroundings, which unlike the fetal brain is not amenable to the use of a common anatomical coordinate frame such as 2. Deep learning approaches potentially provide a route to solving the problem, but can themselves be impacted by differences in MRI contrast. For structural placental MRI segmentation3 deep learning has previously been combined with conventional post processing steps to improve performance. Here we look at approaches where the contrast aspect of the label training problem is simplified by the use of quantitative MRI measures, rather than raw uncalibrated MRI intensities.Methods
Imaging was carried out on a 1.5 T Phillips Achieva/dStream MRI with a 16-channel receiver body coil. Dual echo EPI BOLD data was collected with a repetition time (TR) of between 2.5 and 3s, first echo of 15 to 18ms, second echo between 44.5 and 50.4ms, using a SENSE factor of 2. The in-plane pixel resolution was 3x3mm, slice thickness was 3mm, with no slice gap, and a slice interleave of sqrt(N). The number of slices covering the placenta ranged between 24 and 36, repeated for between 90 and 150 time frames. For each MRI slice, the two echoes acquired were first used to estimate the R2* value at each voxel.A frame from each R2* time series was selected by careful visual and numerical inspection to provide a 3D frame with the least slice to slice motion of placental, maternal and fetal anatomy. A total of 68 of these 3D R2* volumes were used to create training and testing data by careful manual delineation of the placenta by a trained expert, using the rview software (http://rview.colin-studholme.net).
We evaluate the performance of the U-Net architecture6 at voxelwise classification by training on 4 different data schemes: full 3D images, full 2D image slices, patches of 3D images, and patches of 2D images. We used these schemes with the aim to study the benefits patchwise training has for artificially increasing data quantity and to investigate the effectiveness of learned 2D features to that of 3D features. Using 2D slices for training has the added benefit that slices would be robust to interslice motion. Importantly, we make use of the quantitative nature of the R2* data to limit the range of intensity values within our images to that of the placenta tissue in order to simplify the label learning problem. Since each MR image has different dimensions, we also standardize the 3D voxel data by padding with reflections to create 3D images with the same dimensions across all the data for suitable model training and testing. No further data augmentation or modification to the network architecture was used in order to reduce influence on our 4 schemes. Each model was trained for at most 50 epochs with a patience of 4 to avoid overfitting. With that, we evaluated the methods using a 5 fold cross validation for each of the data schemes and use the average Dice score of the 5 folds as our metric for performance.
Results
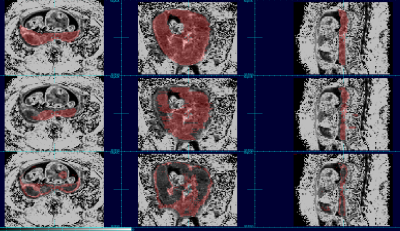
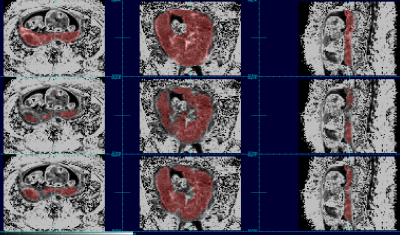
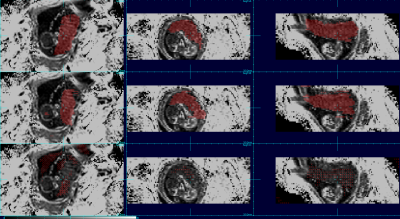
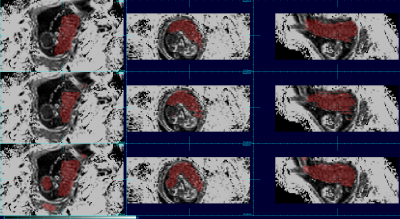
The aggregated results comparing automated to manual maps using DICE coefficients show that the order of the best data schemes are as follows: the set of 3D patches (Dice score: 0.808), the set of 2D patches (Dice score: 0.777), the set of full sized 2D images (Dice score: 0.742) and finally the set of full sized 3D images (Dice score: 0.168). Figures 1 and 2 show the placenta map of an example subject using 4 schemes compared to a manual ground truth; and figures 3 and 4 show examples for a case at a different gestational age. In figures 1 and 2, we can see that results using full training 3D produces significant errors, also apparent in figures 3 and 4, while the patch based schemes perform significantly better.Discussion and Conclusion
We observed that networks trained on full sized 3D R2* images produce significantly greater errors presumably due to the limited examples for training and the greater complexity within the larger volume, limiting the ability to generalize. Reducing the complexity of the scene to be learned by using patches dramatically improves performance. However, 3D patches outperform 2D patches when there is limited between slice motion, but further experimentation may be required for cases of larger motion. Overall, experiments show that patch based training on quantitative R2* maps provides promising overall performance for automated BOLD time series placenta extraction, without resorting to post-processing methods. The methods may be improved further with larger and richer training datasets. The use of other quantitative MRI contrasts may provide a route to improving deep learning performance in other MRI analysis problems.Acknowledgements
The work contributing to this publication was primarily funded by NIHGrants R01 EB017133 and R01 NS055064.References
1. Dighe, Manjiri and Kim, Yun Jung and Seshamani, Sharmishtaa and Blazejewska, Ania I and Mckown, Susan and Caucutt, Jason and Gatenby, Christopher and Studholme, Colin, “Regional placental blood oxygen level dependent (BOLD) changes with gestational age in normally developing pregnancies using long duration R2* mapping in utero”, Proceedings of SPIE Medical Imaging, Image processing 2016
2. C. Studholme, C Kroenke, M. Dighe, "Motion corrected MRI differentiates male and female human brain growth trajectories from mid-gestation", Nature Communications, 11, 3038, June 2020.
3. Torrents-Barrena, Jordina and Piella, Gemma and Masoller, Narcis and Gratacos, Eduard and Eixarch, Elisenda and Ceresa, Mario and Ballester, Miguel Angel Gonzalez, "Fully automatic 3D reconstruction of the placenta and its peripheral vasculature in intrauterine fetal MRI", Medical Image Analysis, 2019, 54
4. Schabel, Matthias C and Roberts, Victoria HJ and Lo, Jamie O and Platt, Sarah and Grant, Kathleen A and Frias, Antonio E and Kroenke, Christopher D, "Functional imaging of the nonhuman primate Placenta withendogenous blood oxygen level--dependent contrast", Magnetic Resonance in Medicine, 2015
5. Zhu, Meng Yuan and Milligan, Natasha and Keating, Sarah and Windrim,Rory and Keunen, Johannes and Thakur, Varsha and Ohman, Annika and Portnoy, Sharon and Sled, John G and Kelly, Edmond and others,"Thehemodynamics of late-onset intrauterine growth restriction by MRI", American Journal of Obstetrics and Gynecology, 2016, 214
6. Olaf Ronneberger, Philipp Fischer, Thomas Brox, “U-Net: Convolutional Networks for Biomedical Image Segmentation”, MICCAI 2015 pp 234
Figures