0707
Longitudinal Placental Blood Volume Measurements on Zika-Infected Rhesus Macaques Using Variable Flip Angle T1 Mapping1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Wisconsin National Primate Research Center, University of Wisconsin - Madison, Madison, WI, United States, 3Comparative Biosciences, University of Wisconsin - Madison, Madison, WI, United States, 4Obstetrics & Gynecology, University of Wisconsin - Madison, Madison, WI, United States, 5Pathology, Oregon Health & Science University, Portland, OR, United States, 6Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 7Obstetrics and Gynecology, University of Wisconsin - Madison, Madison, WI, United States, 8Obstetrics and Gynecology, University of South Florida, Tampa, FL, United States, 9Radiology, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Adequate maternal blood supply is an important factor to maintain placental health, and placenta vascular markers may be predictive of pregnancy outcomes. Here, we report longitudinal quantitative results of maternal fractional, regional, and total blood volume measurements in rhesus macaque placenta across gestation ages using Ferumoxytol-enhanced variable flip angle (VFA)-T1 mapping. We observe regional heterogeneity in fractional blood volume and increased maternal placental blood volume throughout pregnancy.
Introduction
Utero-placental vasculature adaptation during pregnancy plays a crucial role in delivering sufficient nutrient and oxygen exchange and pregnancy outcomes [1-2]. Placental blood volume measurements might hold potential to detect and predict abnormal placental development. MRI has been successfully implemented to estimate fractional blood volume (FBV) in pregnant mice using blood-pool liposomal gadolinium-based contrast agents (GABA) [3]. However, safety concerns and modeling complexities limit the use of GABA in humans. Alternatively, iron based Ferumoxytol is used in pregnancy for the treatment of anemia, and more recently as an MR contrast agent. In this pilot study, we investigated the use of steady state, Ferumoxytol enhanced variable-flip-angle T1 (VFA-T1) mapping to measure FBV, maternal placental blood volume (MPBV), and total maternal blood volume (TBV) across multiple gestational ages.Methods
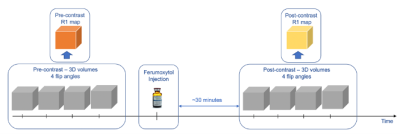
Subjects: Seven pregnant rhesus macaques (body weight 8.20±1.35 kg) were imaged longitudinally across gestational days 64.5±1.9, 100.8±3.9, and 145.3±1.8. Three subjects received a zika virus (ZIKV) injection into the amniotic fluid of 104 plaque forming units (PFU) and one subject received 105 of PFU. Three control rhesus macaques received a saline injection. All injections occurred at gestational age 54.7±1.9 days.MRI Data Acquisition: All subjects were sedated using isoflurane and imaged in right-lateral position on a 3.0 T system (Discovery MR750, GE Healthcare) with a 32-channel phased array coil. T1 mapping was performed with a respiratory-gated center out, 3D radial, VFA T1-weighted spoiled gradient echo (SPGR) sequence (TR = 6.0 ms, TE = 1.2 ms, FOV = 200*200*200 mm3, resolution = 0.78*0.78*0.78 mm3, flip angle = 2°, 6°, 10°, 14°, scan time = 8 min 46 sec). After pre-contrast imaging, Ferumoxytol (4mg/kg with respect to maternal body weight, diluted 5:1 with saline) was intravenously administered with a power injector. Approximately 30 minutes later, the T1 mapping sequence was repeated (Figure 1).
Data processing and analysis: R1 maps were obtained using complex fitting to SPGR signals using an inhouse toolbox in MATLAB (Mathworks, Natick, MA) Pre- and post-contrast scans were registered using non-rigid registration (ANTs [4]). Placental segmentations were conducted manually on the heavily T1-weighted 14° flip angle post-contrast images using an inhouse segmentation tool (MATLAB). In addition, a circular ROI was drawn on a vascular region near the placenta to measure R1 values in blood.
Blood volume calculation: Pre- and post- contrast R1 changes in placental and vascular regions were used to derive the following parameters: (1) FBV, the fractional amount of placental volume occupied by maternal blood, calculated as the ratio between median ΔR1 in placenta and maternal blood [3]; (2) MPBV, the total amount of maternal blood in placenta, calculated as placental volume multiplied by mean FBV, and (3) TBV, calculated as a ratio of amount of ferumoxytol (mol) in placental tissueover concentration of ferumoxyol (M) in blood [5], assuming a relaxivity r1=8.6725s-1mM-1 [6].
Results
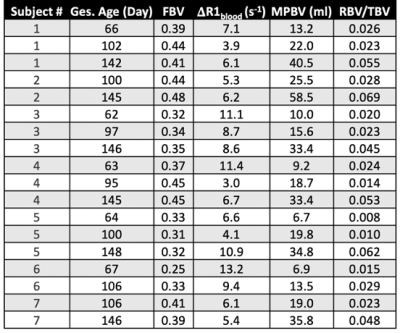
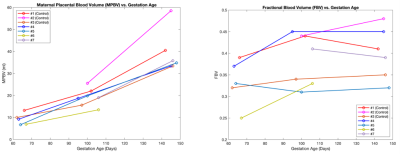
Figure 2 shows representative FBV distribution maps overlaid on top of 6 post-contrast T1-weighted images. FBV exhibits a regionally heterogeneous distribution. Blood volume measurements are summarized in table 1. Median TBV across all scans is 740.3 ml, and median TBV normalized by maternal body weight is 93.4 ml/kg. Blood volume measurements do not show significant differences between control and ZIKV-infected subjects.Figure 3 shows MPBV and FBV measurements vs. gestational age. MPBV for all subjects increased constantly with gestational age, whereas FBV showed heterogeneous trends, with higher degree of heterogeneity during early pregnancy.
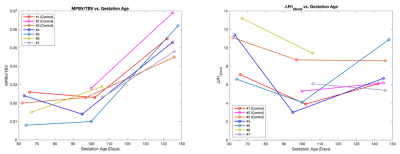
Figure 4 shows longitudinal trends of the ratio of MPBV and TBV, as well as ΔR1 in maternal blood. MPBV/TBV increased consistently for all subjects during later stage of pregnancy, whereas ΔR1 in maternal blood decreased in between first and second gestational time points for all subjects.
Discussion and Conclusion
This pilot study demonstrates the feasibility of placental blood volume measurements using Ferumoxytol-enhanced VFA-T1 mapping in a non-human primate model. To our knowledge, this is the first study to report placental regional blood volume estimates in vivo. FBV was shown to have a regionally heterogeneous distribution, which has been shown to reflect perfusion heterogeneity [7]. Longitudinal analysis shows a heterogeneous trend of FBV across gestational age, whereas MPBV increases consistently with gestational age. The ZIKV infection model was observed to have only very modest placental pathology in a related study [8]. Our study supports modest placental pathology given no significant changes in blood volume were observed.The mean TBV measured in this study from all scans is 50.4% higher than previous TBV measurement in non-pregnant adult rhesus macaques of 62.1 ml/kg using an indicator dilution methodology [9]. This is within the expected range of normal percent of blood volume increase during human pregnancy [10]. However, some of the observed TBV variations are not physiological plausible. The TBV measures dependent on precise knowledge of the amount of Ferumoxytol injected. We suspect that imperfect mixing with saline, incomplete injections solution in line, or extravasation might have occurred in some instances and corrupted TBV estimates. Importantly, MPBV and FBV are more robust measures since exact knowledge of the amount of ferumoxytol injected is not required.
Future studies include detailed longitudinal blood volume analysis with more subjects, including correlations with pregnancy complications and outcomes. These studies are necessary before transition into human experiments.
Acknowledgements
The authors acknowledge funding from NIH grant R21-AI129308 and NIH Human Placenta Project (U01-HD087216), as well as GE Healthcare for their research support.References
[1] Roberts VHJ, Lo JO, Lewandowski KS, et al. Adverse Placental Perfusion and Pregnancy Outcomes in a New Nonhuman Primate Model of Gestational Protein Restriction. Reprod Sci. 2018;25(1):110-119. doi:10.1177/1933719117704907
[2] Hutter, D., Kingdom, J. & Jaeggi, E. Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review. Int. J. Pediatr. 2010, 401323, https://doi.org/10.1155/2010/401323 (2010).
[3] Badachhape, AA, Devkota, L, Stupin, IV, et al. Nanoparticle Contrast-enhanced T1-Mapping Enables Estimation of Placental Fractional Blood Volume in a Pregnant Mouse Model. Sci Rep 9, 18707 (2019). https://doi.org/10.1038/s41598-019-55019-8
[4] Brian BA, Nicholas JT, Gang S, et al. A Unified Image Registration Framework for ITK.Proceedings of the Fifth Workshop on Biomedical Image Registration 2012:266-275.
[5] Pannek K, Fidler F, Kartäusch R, Jakob PM, Hiller KH. Contrast agent derived determination of the total circulating blood volume using magnetic resonance. MAGMA. 2012 Jun;25(3):215-22. doi: 10.1007/s10334-011-0282-7. Epub 2011 Sep 18. PMID: 21928062.
[6] Knobloch G, Colgan T, Wiens CN, Wang X, Schubert T, Hernando D, Sharma SD, Reeder SB. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Invest Radiol. 2018 May;53(5):257-263. doi: 10.1097/RLI.0000000000000434. PMID: 29215401; PMCID: PMC6143390.
[7] Ludwig KD, Fain SB, Nguyen SM, Golos TG, Reeder SB, Bird IM, Shah DM, Wieben OE, Johnson KM. Perfusion of the placenta assessed using arterial spin labeling and ferumoxytol dynamic contrast enhanced magnetic resonance imaging in the rhesus macaque. Magn Reson Med. 2019 Mar;81(3):1964-1978. doi: 10.1002/mrm.27548. Epub 2018 Oct 25. PMID: 30357902; PMCID: PMC6715150.
[8] Seiter D, Ludwig KD, Nguyen S, Murphy ME, Anthony KM, Chen R, Morgan TK, Zhu A, Dhyani A, Fain SB, Johnson KM, Golos TG, Wieben O. Quantitative Ferumoxytol Dynamic Contrast Enhanced (DCE) MRI Evaluation of the Placenta after Zika Virus Infection in the Rhesus Macaque. In: Proc 28th Annual Meeting ISMRM.;2020:0584.
[9] Hobbs TR, Blue SW, Park BS, Greisel JJ, Conn PM, Pau FK. Measurement of Blood Volume in Adult Rhesus Macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2015;54(6):687-693.
[10] Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985 Oct;14(3):601-12. PMID: 4075604.
Figures