0704
Endometrial Carcinoma: Assessment of Histological Features Based on Amide Proton Transfer-weighted Imaging and Diffusion Kurtosis Imaging1Department of Radiology, Zhengzhou University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 2Department of Radiology, Henan University People’s Hospital & Henan Provincial People’s Hospital, Zhengzhou, China, 3Department of MRI, The First Affiliated Hospital of Xinxiang Medical University, Weihui, China, 4GE Healthcare, MR Research China, BeiJing, China
Synopsis
Our results showed that both Amide proton transfer-weighted imaging (APTWI) and Diffusion kurtosis imaging (DKI) were effective in the assessment of endometrial carcinoma in terms of clinical type, histological grade, subtype, and Ki-67 index.
Introduction
Endometrial carcinoma (EC) is a common malignant tumor of the female reproductive system, with high morbidity and mortality [1]. Conventional Magnetic resonance imaging (MRI) often cannot well reflect the microscopic information of lesions, which makes it difficult to provide more detailed guidance for diagnosis and treatment. Amide transfer-weighted imaging (APTWI) is a molecular imaging technology that can achieve noninvasive quantitative assessment of mobile proteins and polypeptides concentrations in tissues without the use of contrast agents [2]. Diffusion kurtosis imaging (DKI) accurately describes the diffusion characteristics of water molecules in tissue; thus, it has high sensitivity concerning reflecting the complexity of the microstructure [3]. At present, only a few small sample studies have separately reported that APTWI [4] and DKI [5] related parameters can be preliminarily used as imaging markers for the assessment of EC histological grade. This study aimed to investigate APTWI and DKI in the evaluation of EC in terms of clinical type, histological grade, subtype, and Ki-67 index, and to compare which technique has a better performance.Material and Methods
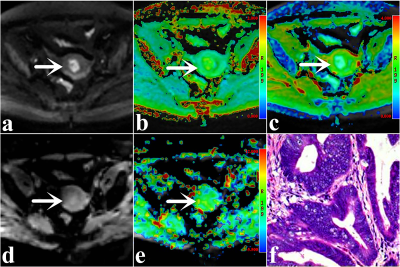
A total of 88 enrolled subjects were classified into the following groups: type Ⅰ (grade 1, 2 endometrioid adenocarcinoma (EA)) and type Ⅱ (grade 3 EA and non-EA (clear cell and serous carcinoma)) EC groups, EA (grade 3) and non-EA groups, and low grade (grade 1, 2) and high grade (grade 3) EA groups. A 3.0-T MR scanner (Discovery MR750, GE Healthcare) with a 16-channel phased-array body coil was performed. First, axial T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and diffusion-weighted imaging (DWI) scans were performed. Next, regarding the above images, the slices where a tumor appeared to be present were selected as the scan sections for APTWI and DKI. APTWI was performed by using a saturation power level of 2.0 μT and a saturation pulse (Tsat) with a duration of 0.5 s. A total of 52 frequencies, including 49 offsets ranging from - 600 to + 600 Hz with an interval of 25 Hz and a frequency 5000 Hz (3 times) far from the resonant frequency, were used for the APTWI and z-spectrum scans for signal normalization. The water saturation shift reference (WASSR) was applied for B0 correction. DKI was set with 5 b values (0, 500, 1000, 1500, and 2000 s/mm2) and 30 diffusion directions. The ROIs, excluding areas with large vessels, hemorrhagic, calcified, cystic, and necrotic, were drawn along the edge at the maximum cross-section of the tumor.MedCalc 15.0 and SPSS 23.0 were employed for statistical analyses. The independent sample t-test was applied for between-group comparison. The ROC curve and Delong test were performed to evaluate and compare the diagnostic performance of each parameter. The Spearman rank and Pearson correlation were employed to describe the correlation of each parameter with histologic grade and Ki-67 index, respectively. Results with P < 0.05 were considered to be significant.
Results
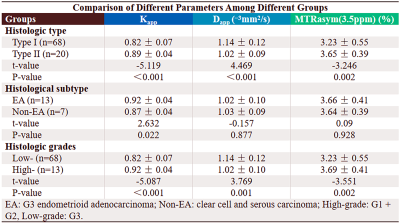
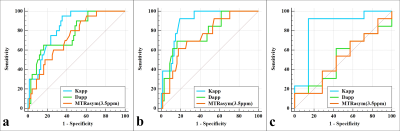
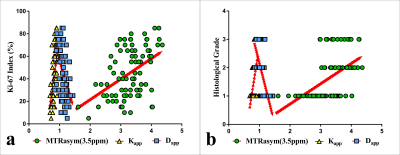
The Dapp was higher, and the Kapp and MTRasym (3.5 ppm) were lower in the type I group than in the type II group . Although the Kapp was higher in the EA group than in the non-EA group (P all < 0.05), the difference in Dapp and MTRasym (3.5 ppm) between the two groups were not significant. Additionally, the Dapp was higher, and the Kapp and MTRasym (3.5 ppm) were lower in the low-grade group than in the high-grade group (P all < 0.05). Regarding the identification of type I and II groups, the AUCs of Kapp, Dapp, and MTRasym (3.5 ppm) were 0.837, 0.774, and 0.732, respectively, but the difference between that of each parameter was not significant. Regarding the identification of EA and non-EA, only the AUC of the Kapp was significant (AUC = 0.846, P = 0.003). Based on a comparison of high-grade versus low-grade groups, the AUCs of Kapp, Dapp, and MTRasym (3.5 ppm) were 0.895, 0.781, and 0.749, respectively, and the difference in AUC between Kapp and MTRasym (3.5 ppm) was significant (Z = 2.031, P = 0.042) . The correlation between each parameter and histological grade and the Ki-67 index was as follows: Kapp, r = 0.759, 0.624; Dapp, r = - 0.704, -0.507; and MTRasym (3.5 ppm), r = 0.555, 0.462. (Figures 1-4).Discussion
Compared with type I EC, low-grade EA, and low Ki-67 EA groups, type II EC, high-grade EA, and high Ki-67 EA groups all have an increase in cellular density, nuclear atypia, microvessel density (MVD), and microscopic necrosis[6, 7, 8], which not only can improve the contents of mobile proteins and polypeptides, also can increase the diffusion deviation of water molecules and limit the diffusion velocity of water molecules, leading to changes in signal intensity (SI) of APTWI and DKI. EA is mainly characterized by high dysplasia of glandular cells, whereas serous and clear-cell carcinoma (non-EA) display dense papillary and solid lamellar growth, respectively[9]. These differences in cell type and growth mode might be the reason for the difference in Kapp between the two groups.Conclusion
Both DKI and APTWI related parameters have potential as imaging markers for estimating the histological features of EC, with DKI having superior performance.Acknowledgements
The National Key R&D Program of China (2017YFE0103600), the Henan Medical Science and Technology Research Program (2018020357 and 2018020367), the National Natural Science Foundation of China (81720108021 and 31470047), and Zhongyuan Thousand Talents Plan Project - Basic Research Leader Talent (ZYQR201810117).References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1):7-34.
2. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085-1090.
3. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-1440.
4. Takayama Y, Nishie A, Togao O, et al. Amide Proton Transfer MR Imaging of Endometrioid Endometrial Adenocarcinoma: Association with Histologic Grade. Radiology. 2018;286(3):909- 917.
5. Yamada I, Sakamoto J, Kobayashi D, et al. Diffusion kurtosis imaging of endometrial carcinoma: Correlation with histopathological findings. Magn Reson Imaging. 2019;57:337-346.
6. Zaino RJ, Kurman RJ, Diana KL, Morrow CP (1995) The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 75:81-86.
7. Dobrzycka B, Mackowiak-Matejczyk B, Kinalski M, Terlikowski SJ (2013) Pretreatment serum levels of bFGF and VEGF and its clinical significance in endometrial carcinoma. Gynecol Oncol 128:454-460.
8. Fukunaga T, Fujii S, Inoue C et al (2015) Accuracy of semiquantitative dynamic contrast-enhanced MRI for differentiating type II from type I endometrial carcinoma. J Magn Reson Imaging 41:1662-1668.
9. Rutgers JK (2015) Update on pathology, staging, and molecular pathology of endometrial (uterine corpus) adenocarcinoma. Future Oncol 11:3207-3218.
Figures