0702
Diffusion MRI study of chemoradiation treatment response in patients with HPV positive oropharyngeal carcinoma1Radiology, Weill Cornell Medical College, New York, NY, United States, 2Radiology, New York University School of Medicine, New York, NY, United States, 3Radiation Oncology, New York University School of Medicine, New York, NY, United States
Synopsis
In this study, we evaluated diffusion and kurtosis time-dependence for HPV-positive oropharyngeal squamous cell carcinoma before and during chemo-radiation treatment over a wide range for longer diffusion times (200-700 ms). The patients with less than 40% nodal volume shrinkage had significantly higher diffusivity at pretreatment and lower kurtosis at week4 than the patients with more than 40% nodal volume shrinkage. The water exchange times were 68-80 ms without a significant difference between the groups. This study demonstrates the feasibility of using diffusion MRI at relatively long diffusion times to predict and evaluate the response to chemo-radiation therapy.
Introduction
Recent studies showed that a subgroup of head and neck cancer patients with human-papilloma virus (HPV)-positive oropharyngeal squamous cell carcinoma (OP-SCC) have significantly better prognosis (1). These data lead to important considerations to de-intensify treatment for this low-risk, younger population in order to reduce acute and chronic toxicity without compromising disease control. Diffusivity and diffusional kurtosis have been proposed as imaging markers to assess cell viability to evaluate the early treatment response (2-6). However, most of previous studies were conducted with short diffusion times (~100 ms), and have not explored the full potential of diffusion MRI to measure specific tissue microstructural properties. In this abstract, we evaluate diffusion and kurtosis time-dependence for HPV-positive OPSCC before and after therapy over a wide range of longer diffusion times (200-700 ms).Methods
The patients were recruited from an ongoing phase II institutional clinical research protocol, “Adaptive de-escalation of radiation therapy dose in HPV-positive oropharyngeal carcinoma (ART) demonstrating favorable mid-treatment response”. During the course of radiotherapy, all patients had a repeat CT scan at four weeks (week 4) of treatment to evaluate patient’s treatment response to chemo-radiation. Patients who had lymph node shrinkage > 40% at week 4 were given a dose de-escalated treatment regimen for a total dose of 60 Gy. Patients who did not meet the criteria received the standard treatment for a total of 70 Gy as initially planned.Eighteen OPSCC patients (6 non-deescalated and 12 deescalated) were imaged on a Siemens 3T PRISMA system using a 20-channel head/neck coil. An in-house developed stimulated echo acquisition mode (STEAM) EPI sequence was used to acquire 5 diffusion times, [t=100,200,300,500,700 ms], over 4 b-shells [b=500,1000,2000,3000 s/mm2] with 3 diffusion directions along x, y, and z axes. The mixing time, tm, was [80,180,280,480,680] ms varying with t. Other parameters include, TR=5000ms, TE=66 ms, resolution=1.5x1.5x4.0 mm, FOV=190 mm, partial Fourier 6/8, and GRAPPA with R=2. Each patient was imaged twice: once before initiating chemo-radiation therapy and then 4-weeks after starting the therapy.
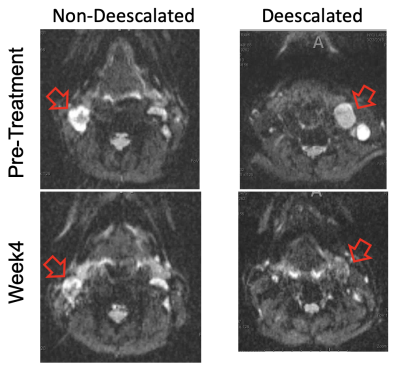
Each set of images was affine registered over all b and t. Figure 1 shows example b=0 images for one non-deescalated and one deescalated patient. Following post-processing, multi-slice regions of interest (ROI) were drawn for the largest lymph node in each patient. For each voxel in the ROIs, diffusion and kurtosis maps were generated via a model-based method assuming that for the range of t in this experiment, diffusion through the tumor can be considered Gaussian where D(t) remains constant. In this regime, D is sensitive towards exchanging volume fractions, ve, whose water exchange time13 could be determined by modeling K(t) using the Karger model (7):
$$ D=(1-v_e ) D_e+v_e D_i=const$$
$$ K(t)=K_∞+K_0 (2τ_{ex})/t [1-τ_{ex}/t (1-e^{(-t⁄τ_{ex} ) })]$$
where K_∞ marks the floor of diffusion kurtosis pertaining to the intrinsic tissue heterogeneity. From D and K(t), we can predict the diffusion weighted signal using the cumulant expansion of signal including both D and K:
$$S_p(t,b)=exp(-bD+(1/6) b^2D^2K(t))$$
Sp(t, b) can be linearly scaled to match the actual signal range of diffusion signal Sm(t, b) for each diffusion time. Then, estimation of four parameters is conducted by minimizing the sum of squared differences between the predicted and measured signals for each voxel:
$$(K_0, K_∞, τ_ex,D_c)=argmin ∑_(t,b)(S_p (t,b) (-) S_m (t,b))^2 $$
Results
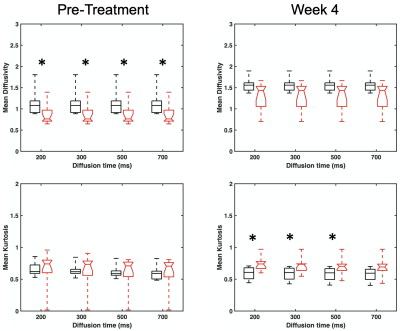
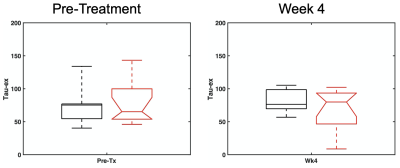
Comparisons of D and K(t) values for different diffusion times were conducted based on the voxels with D < 2.0 um2/ms in order to exclude non-solid part of tumors. Figure 3 shows that the non-deescalated group had significantly (p < 0.05; Wilcoxon rank sum test) higher pretreatment D than the deescalated group. At week 4, the non-deescalated group had significantly (p< 0.05) lower K than the deescalated group. The water exchange time tex of the non-deescalated and deescalated groups did not significantly differ from each other at both time points (76.1 ± 31.9 ms vs 79.4 ± 34.2 ms for pre-treatment and 80.6 ± 18.6 ms vs 68.3 ± 30.1 ms for week 4) (Figure 4).Discussion
The results of this study in terms of D are in agreement with previous studies which showed that lower D at pretreatment was associated with favorable response to chemoradiation treatment. It is remarkable that the similar trend of D can be found among HPV-positive OPSCC patients who are known to have good prognosis in general. In addition, our results suggest that the K at week 4 can be also helpful to identify patients with good response. Further study is required to investigate how both D and K could be used at different treatment time points to reliably predict and evaluate the treatment response. The water exchange times measured in this approach at voxel-level appear to be in the expected range for cancer cells. Future studies can also assess a potential association of the water exchange time as well as other diffusion parameters with long-term outcome of the treatment.Conclusion
This study with HPV-positive OPSCC patients demonstrates the feasibility of using diffusion MRI at relatively long diffusion times to predict and evaluate the response to chemo-radiation therapy, and the potential of utilizing these parameters for identifying patients eligible for de-escalation treatment in future studies.Acknowledgements
NIH UG3CA228699, R01CA160620, R01CA219964, R01-EB028774, P41EB017183References
1. Denis F., Garaud P., Bardet E., Alfonsi M., Sire C., Bergerot T.G., Rhein B., Tortochaux J., Calais G., Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2004;22(1):69-76.
2. Padhani A.R., Liu G., Koh D. M., Chenevert T.L., Thoeny H.C., Takahara T., Dzik-Jurasz A., Ross B.D., Cauteren M.V., Collins D., Hammoud D.A., Rustin G.J.S., Taouli B., Choyke P.L., Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11(2):102-125.
3. Kim S., Loevner L., Quon H., Sherman E., Weinstein G., Kilger A., Poptani H., Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clinical cancer research : an official journal of the American Association for Cancer Research 2009;15(3):986-994.
4. Thoeny H. C., and Ross B. D., Predicting and Monitoring Cancer Treatment Response with Diffusion-Weighted MRI. Journal of Magnetic Resonance Imaging 2010;32(1):2-16.
5. Jansen J. F., Stambuk H.E., Koutcher J.A., Shukla-Dave A., Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: A feasibility study. AJNR American journal of neuroradiology 2010;31(4):741-748.
6. Goshima S., Kanematsu M., Kondo H., Yokoyama R. et al. Diffusion kurtosis imaging to assess response to treatment in hypervascular hepatocellular carcinoma. AJR American journal of roentgenology 2015;204(5):W543-549.
7. Jensen J.H., Helpern J.A., Ramani A., Lu H., Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine 2005;53(6):1432-40.
Figures