0701
Looking Inside a Voxel through the Lenses of Non-Gaussian Diffusion MRI: Correlation between Imaging- and Histology-based Tissue Heterogeneity1Center for Magnetic Resonance Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Department of Electrical and Computer Engineering, University of Illinois at Chicago, Chicago, IL, United States, 4Research Resources Center, University of Illinois at Chicago, Chicago, IL, United States, 5Department of Radiology, Northwestern University, Chicago, IL, United States, 6Department of Computer Science, University of Illinois at Chicago, Chicago, IL, United States, 7Department of Pathology, University of Illinois at Chicago, Chicago, IL, United States, 8Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Studies on tissue structural heterogeneity have been the focus of a growing number of non-Gaussian diffusion models, such as the continuous-time random-walk (CTRW) model. Establishing a correlation between the voxel-level CTRW parameters and the microscopic tissue heterogeneity from gold-standard histology, however, has been challenging due to the lack of quantitative measure of histopathological heterogeneity and different spatial scales. We establish a one-to-one correspondence between imaging-based tissue heterogeneity revealed by CTRW parameters and histology-based tissue structural heterogeneity predicted by a machine-learning classifier to address an overarching question: “Can we look inside a voxel noninvasively through the lenses of the CTRW model?”.
Introduction
Tissue heterogeneity has been regarded as a hallmark of tumor malignancy1-3. While histological analysis is the gold standard for its assessment, probing tissue structural heterogeneity has been the main motivation of a number of recent diffusion-weighted MRI (DWI) studies4-10. Among several advanced diffusion models, continuous-time random-walk (CTRW) model6,7 unifies Gaussian and non-Gaussian diffusion descriptions; and characterizes non-Gaussian diffusion dynamics in terms of diffusion waiting time and jump length distributions. The spatial and temporal heterogeneity parameters proposed by the CTRW model have been linked to the underlying microscopic tissue heterogeneity6-10. In this study, we aim at establishing a rigorous correlation between imaging-based tissue heterogeneity revealed by CTRW parameters and histology-based tissue structural heterogeneity to help answer this question: “Can we reveal intra-voxel tissue heterogeneity noninvasively through the lenses of the CTRW model?”. We address this question by 1) developing a machine-learning (ML) classifier to quantify the microscopic tissue heterogeneity from histology and 2) demonstrating the correspondence between the CTRW parameters and histology-based heterogeneity level probability maps on both normal and glioma brain specimens.Methods
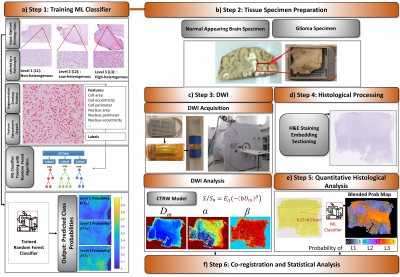
The study workflow consists of six steps (Fig.1):ML Classifier Training (Fig.1a): Training data consisted of 30 digitized histology slides from 7 normal and 9 glioma brain samples, which were labeled with three levels of microscopic heterogeneities: L1-L3. For each level, 50 prototype image patches (1000×1000 pixels) were used. 33 statistical features were generated to characterize nuclei and surrounding structures in Qupath. A random forest algorithm was trained to predict the probability of a given pixel to have a heterogeneity level of L1, L2, or L3 ($$$p(L_i ),i=1,2,3$$$ and $$$\sum_{i=1}^3p(L_i ) =1$$$) in Python's Scikit-Learn. Model performance was validated with 10-fold cross-validation.
Tissue Specimen Preparation (Fig.1b): The specimens from normal appearing human brain (NAs) and postmortem human glioma (GLs) were fixed in paraformaldehyde and individually enclosed into tissue embedding cassettes (~3×5 cm2 in size).
DWI Acquisition and Analysis (Fig.1c): The specimens were scanned on a 9.4T Agilent MRI scanner in the cassettes placed in tubes filled with saline (for NAs) or Fluorinert (for GLs), a proton-free susceptibility-matching fluid. The protocol included T1 and T2 imaging, and DWI with 16 b-values (0-5000 s/mm2; TR/TE=2000/28ms, slice thickness=0.3mm, Δ/δ=18/2.5ms, in-plane resolution=0.25×0.25mm2). Trace-weighted diffusion images were analyzed using the CTRW model7,$$S/S_0=E_α (-(bD_m )^β),\tag{1}$$ where Dm is an anomalous diffusion coefficient, Eα is a Mittag-Leffler function, and α and β are temporal and spatial diffusion heterogeneity parameters, respectively. A nonlinear least-squares algorithm was used to estimate the CTRW parameters.
Histological Processing (Fig.1d): After MRI, the specimens were H&E stained; and sectioned into 50 histology slides, consisting of five 5μm-thick histology slides corresponding to each imaging slice
Quantitative Histological Analysis (Fig.1e): Each digitized histology slide was partitioned into small tiles with the same spatial resolution as the diffusion-weighted images. After feature extraction, $$$p(L_i )$$$s were predicted for each tile by the trained ML classifier. A “blended” predicted probability map was created by displaying the probability value of the “assigned” heterogeneity level (AHL; i.e., the level with the highest probability) with a specific color range.
Co-registration and Statistical Analysis (Fig.1f): The histology-based heterogeneity level probability maps and diffusion-weighted images were co-registered through an affine transformation. The CTRW parameter values were grouped into ROIs drawn according to their AHLs (Figs.3f and 4g). Statistical analysis was performed by a Mann-Whitney-U-test for the NAs where the ML classifier resulted in two types of AHL (L2 and L3), and by a one-way ANOVA analysis followed by post-hoc tests for the GLs.
Results
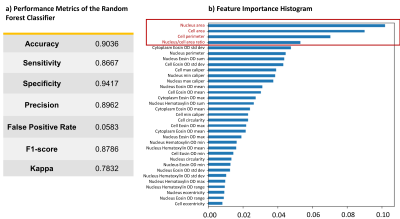
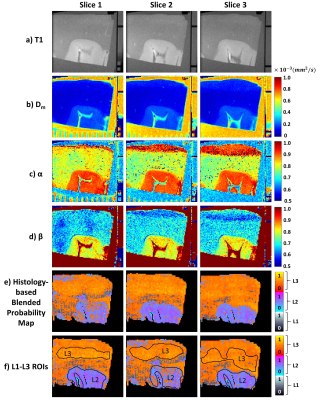
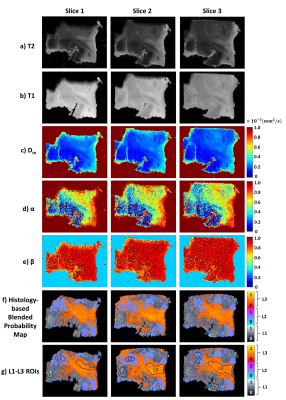
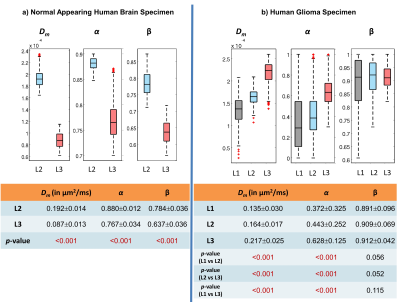
The ML classifier performance results are summarized in Fig.2a. The top four most important features were determined as nucleus area, cell area and perimeter, and nucleus/cell area ratio as in Fig.2b. In the NAs, the regions with lower CTRW parameter values (i.e., white matter; Figs.3b-3d) were found to be mostly assigned to L3 (red-yellow pixels; Fig.3e) while the voxels with higher CTRW parameter values (i.e., gray matter; Figs.3b-3d) were mostly classified as L2 (blue-pink pixels; Fig.3e). In the GLs, CTRW parameters Dm and α (Figs. 4c and 4d) showed contrast correlation with the histology-based blended probability maps (Fig.4f), while β exhibited virtually no image contrast (Fig.4e). Unlike in the NAs case, the regions with higher Dm and α values were assigned to a higher heterogeneity level (L3; red-yellow pixels; Fig.4f) than those with lower values (L1; black-white pixels; Fig.4f). These observations were substantiated in the statistical analysis (Fig. 5). The CTRW parameters showed significant differences (p<0.05) in all comparisons among the AHLs in both specimen types except for β in the GLs.Discussion and Conclusion
We have rigorously demonstrated a one-to-one correspondence between tissue heterogeneity probed by the CTRW model and tissue heterogeneity obtained from histology. Our results have shown a positive correlation between the CTRW parameters and histology-based heterogeneity quantified by the ML classifier in the NAs while the results were reverse in the GLs. Although it has been shown that Fluorinert does not impact the cellular integrity of normal tissue and does not have detrimental effects on histological analysis11,12, its effect on the diffusion dynamics of glioma tissue has not been well reported. Nevertheless, this study provides initial insights into using DWI models for non-invasive characterization of intra-voxel tissue heterogeneity.Acknowledgements
This work was supported in part by the National Institutes of Health (5R01EB026716-01 and 1S10RR028898-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Peter S. LaViolette and Medical College of Wisconsin Tissue Bank for providing the normal appearing human brain specimens.References
[1] Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–37.
[2] Bedard PL, Hansen AR, Ratain MJ, et al. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64.
[3] Fletcher CDM. The evolving classificiation of soft tissue tumors – an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2-11.
[4] Kwee TC, Galban CJ, Tsien C, et al. Intravoxel water diffusion heterogeneity imaging of human high-grade gliomas. NMR Biomed. 2010;23(2):179–187.
[5] Szczepankiewicz F, van Westen D, Englund E, et al. The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE). Neuroimage. 2016;142:522-532.
[6] Ingo C, Magin RL, Colon-Perez L, Triplett W, Mareci TH. On random walks and entropy in diffusion‐weighted magnetic resonance imaging studies of neural tissue. Magn Reson Med. 2014;71:617–627.
[7] Karaman MM, Sui Y, Wang H, et al. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med. 2015; 76:1149-1157.
[8] Sui Y, He W, Damen FW, et al. Differentiation of low- and high- grade pediatric brain tumors with high b-value diffusion weighted MR imaging and a fractional order calculus model. Radiology. 2015;277(2):489–496.
[9] Sui Y, Xiong Y, Xie KL, et al. Differentiation of low- and high-grade gliomas using high b-value diffusion imaging with a non-Gaussian diffusion model. Am J Neuroradiol. 2016; 37:1643-1649.
[10] Tang L, Sui Y, Zhong Z, et al. Non-Gaussian diffusion imaging with a fractional order calculus model to predict response of gastrointestinal stromal tumor to second-line sunitinib therapy. Magn Reson Med. 2018; 79(3):1399-1406.
[11] Iglesias JE, Crampsie S, Strand C, et al. Effect of Fluorinert on the histological properties of formalin-fixed human brain tissue. J Neuropathol Exp Neurol. 2018; 77(12): 1085–1090.
[12] Hyare H, Powell C, Thornton J, et al. Perfluoropolyethers in magnetic resonance microscopy: Effect on quantitative magnetic resonance imaging measures and histological properties of formalin-fixed brain tissue. Proc Int Soc Mag Res Med 2008;1719.
Figures