0694
Comparison of free-breathing self-gated continuous IR spiral T1 mapping: dual flip angle versus Bloch-Siegert B1-corrected techniques1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 3Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 5Cardiology, Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
Synopsis
We propose a technique to acquire accurate B1 and T1 maps in a free-breathing cardiac self-gated continuous Look-Locker, inversion-recovery acquisition. Data are acquired using a single spiral interleaf, rotated by the golden-angle in time. During the first 2 seconds, off-resonance Fermi pulses are applied to generate a Bloch-Siegert shift B1 map, and the later data are acquired with an inversion RF pulse applied every four seconds to create T1* map. The final T1 map is generated with the B1 map and T1* map by using a look-up table to account for slice profile effects yielding more accurate T1 values.
Introduction
Cardiac T1 maps have demonstrated the ability to assess both focal and diffuse myocardial processes in cardiomyopathy 1,2. However, to use a continuous Look-Locker acquisition, where cardiac self-gating can be achieved, both B1 and slice profile effects3,4 need to be considerated to quantify T1. Recently, we proposed a technique to obtain T1 and flip-angle-scale maps in a single free-breathing self-gated continuous inversion-recovery (IR) acquisition using two excitation flip angles 5 (2FAs). To further improve T1 mapping accuracy, we propose a Bloch-Siegert B1-corrected single flip angle acquisition under free-breathing and cardiac self-gating, and compare it with the previous technique.Method
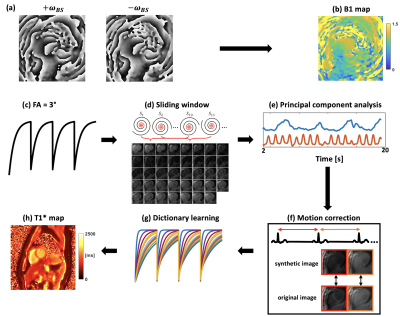
Acquisition strategyAs shown in Figure 1, for Bloch-Siegert shift (BS) B1 mapping 6, data were acquired continuously with golden-angle rotated spiral trajectories for 2 seconds. An off-resonance Fermi pulse was applied between the slice rewinder and the readout-gradient lobes. To minimize motion effects during acquisition, positive and negative off-resonance pulses are interleaved for each spiral trajectory. For T1* mapping, following an inversion-recovery (IR) RF pulse, golden-angle spiral trajectories are acquired continuously over 4 seconds using a spoiled-GRE pulse sequence. This pattern is repeated 4 times. In the 2FAs approach, the IR acquisition is repeated with a second flip angle.
Reconstruction
For the BS B1 map, images were reconstructed using NUFFT 7 and Walsh coil combination 8. Positive and negative BS phase images (Figure 2a) were then extracted to calculate the B1 map (Figure 2b). For the T1* map, self-gating cardiac triggers were extracted from a sliding-window heart image navigator (Figure 2d,e). Respiratory motion correction was performed by rigidly registering the original image with its corresponding synthetic image that is generated from principal component analysis (PCA), as they share similar image contrast (Figure 2f). Then, the T1* map was obtained by fitting the 3-parameter model using dictionary learning (Figure 2g) reconstructed images as described previously 5.
In order to account for slice profile and B1 effects, firstly the dictionaries were generated by simulating 200 isochromats across the actual slice profile (time-bandwidth [TBW] = 5.4). Then, 200 isochromats were simulated with the proposed acquisition parameters across the slice profile for a range of T1 values from 200 ms to 2500 ms and B1 scales from 0.4 to 1.2 to create a look up table including slice profile effects. Finally, a T1 map was generated by using the B1 map, T1* map as well as the look-up table.
Imaging experiments
Imaging experiments were performed at 3T in the T1MES phantom 9 and five human subjects. The proposed strategy (1FA+B1) and the previous technique (2FAs) were performed and compared to IR-SE, MOLLI 10 and SASHA 11 T1 maps. For BS B1 mapping, parameters included: Fermi pulse duration = 8 ms, off-resonance shift = ±4 kHz, KBS = 79.73 rad/G2, B1,peak = 0.0544 G; FOV = 340 mm, spatial resolution = 10 × 10 × 8 mm3, TR/TE = 40.2/9.14 ms, flip angle = 15°. For T1* mapping, parameters included: TR/TE = 8.35/1.45 ms, RF pulse TBW = 5.4, FOV = 340 mm, spatial resolution = 1.5 ×1.5 × 8 mm3, flip angle = 3°. The T1 values were compared by drawing region of interests (ROIs) in different tubes (phantom) and the regions of myocardium and blood pool (human subjects).
Results
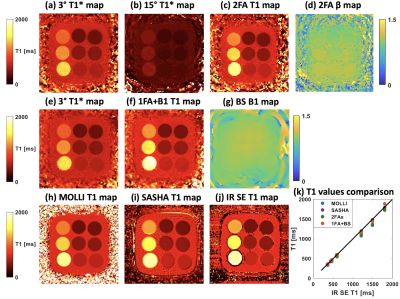
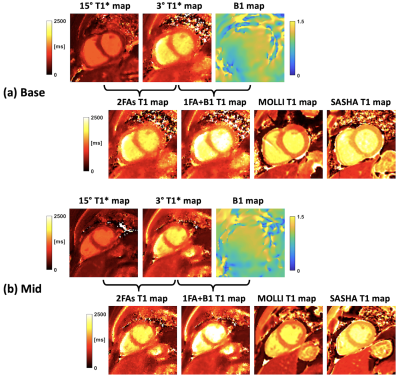
Figure 3 shows the phantom results of 1FA+B1 and 2FA techniques, compared to results from MOLLI, SASHA and IR-SE. The phantom T1 values of 9 tubes from 1FA+B1 and 2FAs are in close agreement with the IR-SE results (Figure 3k). Figure 4 shows results from one human subject for short-axis basal (Figure 4a) and middle (Figure 4b) slices for 2FAs and 1FA+BS techniques, compared to MOLLI and SASHA T1 maps. Table 1 compares the T1 values for myocardium and blood pool among the four mentioned techniques. The myocardium T1 values for 1FA+BS were more similar to those from SASHA T1 maps, which are known to more closely match IR-SE as compared to MOLLI, which tends to underestimate T1 values, especially in pre-contrast studies.Discussion and Conclusion
We developed a strategy to acquire B1 and T1 maps in a free-breathing, continuous inversion-recovery spiral acquisition, providing another method to measure T1 using a continuous Look-Locker acquisition as compared to the previously proposed dual excitation flip angle technique. For in-vivo studies, myocardium T1 values from the proposed method were more similar to the standard breath-held SASHA technique than those from MOLLI, demonstrating increased accuracy in measuring T1. However, the T1 values in blood pool from the proposed technique suffer from some in-flow effects.Acknowledgements
This work was supported by NIH R01 HL131919, Coulter Foundation Grant and AHA pre-doctoral fellowship.References
1. Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, Sibley CT, Bluemke D a. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012;14:90.
2. Jr ANA, Melo MDT De, Lima CR, Jr RND, Nomura CH, Salemi VMC, Jerosch-herold M, Parga JR. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. Eur Hear J - Cardiovasc Imaging. 2018;1–8.
3. Yarnykh VL. Optimal radiofrequency and gradient spoiling for improved accuracy of T1 and B1 measurements using fast steady-state techniques. Magn Reson Med. 2010;63:1610–1626.
4. Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: Searching for common ground. Magn Reson Med. 2015;73:514–522.
5. Zhou R, Weller DS, Yang Y, Wang J, Mugler JP, Salerno M. Free-breathing continous cine and T1 mapping acquisition using a motion-corrected dual flip angle inversion-recovery spiral technique at 3T. In: ISMRM 2020. 2020.
6. Sacolick LI, Wiesinger F, Hancu I, Vogel MW. B1 mapping by Bloch-Siegert shift. Magn Reson Med. 2010;63:1315–1322.
7. Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. J Magn Reson. 2007;188:191–195. 8. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690.
9. Captur G, Gatehouse P, Kellman P, Heslinga FG, Keenan K, Bruehl R, Prothmann M, Graves MJ, Chiribiri A, Ittermann B, Pang W, Nezafat R, Salerno M, Moon JC. A T1 and ECV phantom for global T1 mapping quality assurance: The T1 mapping and ECV standardisation in CMR (T1MES) program. J Cardiovasc Magn Reson. 2016;18:1–4.
10. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (MOLLI) for high-resolution T 1 mapping of the heart. Magn Reson Med. 2004;52:141–146.
11. Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. Magn Reson Med. 2014;71:2082–2095.
Figures