0690
3D Whole-ventricle, Free-Breathing, Non-ECG, T1-T2-B1+ Mapping and Cine Imaging with Cardiac MR Multitasking1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 3Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Siemens Medical Solutions Inc., Los Angeles, CA, United States
Synopsis
Conventional cardiovascular MR (CMR) exams are relatively inefficient and demanding for patients because 1) they rely on methods that necessitate breath-holding or intermittent pauses (via gating) to compensate for motion, 2) images are acquired as a series of 2D slices often with large gaps. We propose a single 3D free-breathing acquisition without ECG requirements, providing motion resolved, quantitative T1 and T2 cine mapping with whole-ventricle coverage with high resolution and no slice gaps. The 3D Multitasking framework additionally incorporates a B1+ component, critical for accurate T1 measurement at 3T. This technique is preliminarily validated both in phantoms and healthy volunteers.
Introduction
Myocardial T1 and T2 mapping are used to assess focal and diffuse cardiac pathologies. Cardiac cine MRI is also the gold standard for assessing cardiac motion and function. Single-acquisition 3D joint T1/T2 mapping with simultaneous cine motion resolution would offer significant efficiency benefits over standard gated, 2D, contrast-specific, serial imaging acquisition. Current free-breathing myocardial multi-parametric mapping techniques1-3 have been reported at 1.5T, and still require ECG triggering or retrospective gating. MR Multitasking has demonstrated T1/T2 cine mapping without ECG gating and breath-holding, but only in 2D slices thus far4. We propose a 3D free-breathing, non-ECG, T1 and T2 cine mapping technique. The non-uniformity of the B1+ transmit field often causes a different flip angle to be experienced by tissue, so our 3D Multitasking framework additionally incorporates a novel B1+ mapping component5 to allow for accurate T1 mapping.Methods
Sequence Design: The proposed sequence uses a prototype 3D stack-of-stars trajectory modified to collect low-rank tensor auxiliary data interleaved with image data. The image data is incremented by a tiny golden angle (i.e., 23.67°). The auxiliary data is acquired every 8th readout. Interleaved IR and T2-IR pulses are employed to generate the T1 and T2 contrasts (i.e., Fig. 1). FLASH excitation alternates between 3° and 10° for each recovery period to allow for B1+ robust T1 mapping5-7.Image Reconstruction: The images acquired in the Multitasking framework can be represented as a 6-way tensor $$$ \boldsymbol{\mathcal{A}} $$$ with voxel location index $$$ \mathbf{r}=(x,y,z) $$$, a T1 recovery dimension, a T2 decay dimension, a cardiac motion dimension, a respiratory motion dimension, and a flip angle dimension. The high correlation along and across multiple time dimensions induces $$$ \boldsymbol{\mathcal{A}} $$$ to be low-rank, which can be factorized as $$$ \boldsymbol{\mathcal{A}}_{(1)}=\mathbf{U} \mathbf{\Phi} $$$, where $$$ \boldsymbol{\mathcal{A}}_{(1)} $$$ is the mode-1 matricization of $$$ \boldsymbol{\mathcal{A}} $$$, $$$ \mathbf{U} $$$ contains spatial coefficient maps, and $$$ \mathbf{\Phi} $$$ contains matricized, separable multi-dimensional temporal basis functions describing T1/T2 relaxation, cardiac motion, respiratory motion, and FLASH readout flip angles. In reconstruction, $$$ \mathbf{\Phi} $$$ was first determined from the HOSVD of the low-rank tensor–completed auxiliary data. $$$ \mathbf{U} $$$ was then recovered by fitting $$$ \mathbf{\Phi} $$$ to the acquired imaging data $$$ \mathbf{d} $$$, according to the following optimization problem:$$ \mathbf{\hat{U}}=\text{arg}\underset{\mathbf{U}}{\operatorname{min}} ||\mathbf{d}-\Omega(\mathbf{FSU\Phi})||_2^2 + R(\mathbf{U}), $$ where $$$ \Omega $$$ is the undersampling operator, $$$ \mathbf{F} $$$ is the nonuniform Fourier transform operator, $$$ \mathbf{S} $$$ is the coil sensitivity operator, and $$$ R(\cdot) $$$ is a regularization functional promoting transform sparsity (in this case, wavelet sparsity of $$$ \mathbf{U} $$$).
T1,T2 Quantification: The native T1, T2 measurements are estimated from the signal model $$ s(A,\beta,B,T_1,T_2)=A \frac{1-e^{-TR/T_1}}{1-e^{-TR/T_1}cos(\beta \alpha)} [1+(Be^{-\tau/T_2}-1)(e^{-TR/T_1}cos(\beta \alpha))^n]\cdot sin(\alpha), $$ with amplitude factor $$$ A $$$, IR/T2-IR pulse efficiency $$$ B $$$, FLASH readout interval $$$ TR $$$, prescribed flip angle $$$\alpha$$$, Look-Locker B1+ factor5 $$$\beta$$$ and recovery time point $$$ n=1,2,..., N$$$, ( $$$ N $$$ is the number of FLASH pulses in a recovery period).
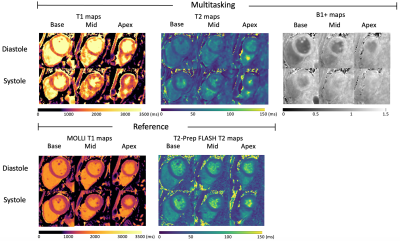
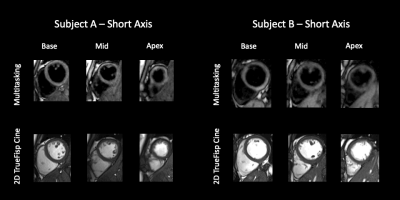
Data Collection: A 16-vial ISMRM NIST phantom and 7 human volunteers (3 male; 4 females) were imaged on a 3T scanner (MAGNETOM Vida, Siemens). Multitasking pulse sequence parameters were: TR/TE=3.6 ms/1.7 ms, recovery period = 2.5 s, spatial resolution=1.4x1.4x8.0 mm3, scan time=9min14s, 20 cardiac bins, 6 respiratory bins. Reference 2D T1 maps with MOLLI and 2D T2 maps with T2-prep FLASH (matched resolution, 1.4x1.4x8.0 mm3) were acquired at both systole and diastole during end-expiration breath-holds at basal, mid, and apical short-axis slices. A full short-axis stack of ECG-gated, breath-held, 2D bSSFP cine scans (1.3x1.3x8.0 mm3, TA=11s per slice) were acquired.
Results
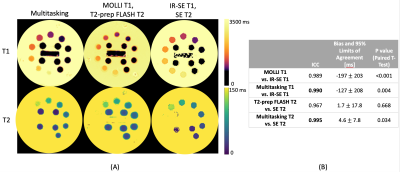
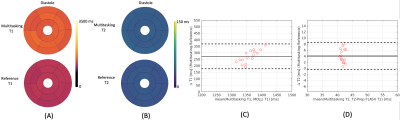
T1 and T2 mapping results from the phantom are shown in Fig. 2. Diastolic and systolic T1, T2, and B1+ mapping results from one subject are shown in Fig. 3, including the basal, mid, and apical short-axis slices from the 3D multitasking sequence and reference 2D MOLLI and T2-prep FLASH sequences. Fig. 4 depicts the summary statistics for segment-wise T1/T2 values in human volunteers. Multitasking T1 maps reported higher myocardial T1 values (1495±47.8ms) than the MOLLI T1 values (1223±16.1ms). Multitasking T2 maps also produced slightly higher myocardial T2 values (43.8±1.3 ms) than the T2-prep FLASH T2 values (39.8±1.3 ms). Fig. 4 also shows the black-blood cine maps from the Multitasking output and the bright-blood cine maps from the bSSFP cine sequence.Discussion
In phantom experiments, Multitasking measurements produced slightly higher ICC against gold standard IR-SE and SE measurements than the MOLLI and T2-prep FLASH sequences did, but all ICCs were >0.96. Although Multitasking produced substantially higher T1 measurements than MOLLI, MOLLI is known to underestimate T1; Multitasking measurements were closer to the reported values from SASHA at 3T8, which is known to be more accurate than MOLLI. Look-Locker B1+ maps showed a lower Look-Locker effect in the blood pool than myocardium, consistent with inflow.Conclusion
3D MR Multitasking is feasible for myocardial T1/T2 mapping and cine imaging in the myocardium at 3T without ECG and breath-holds. Future studies will focus on improving the SNR of T1-T2 cine maps without extending the scan time. Larger cohorts including patients (e.g., cardiomyopathy patients) are necessary for further investigation.Acknowledgements
This work was supported by NIH 1R01EB028146.References
[1] Qi, H., Bustin, A., Cruz, G., Jaubert, O., Chen, H., Botnar, R. M., & Prieto, C. (2019). Free-running simultaneous myocardial T1/T2 mapping and cine imaging with 3D whole-heart coverage and isotropic spatial resolution. Magnetic resonance imaging, 63, 159-169.
[2] Guo, R., Chen, Z., Herzka, D. A., Luo, J., & Ding, H. (2019). A three‐dimensional free‐breathing sequence for simultaneous myocardial T1 and T2 mapping. Magnetic resonance in medicine, 81(2), 1031-1043.
[3] Milotta, G., Bustin, A., Jaubert, O., Neji, R., Prieto, C., & Botnar, R. M. (2020). 3D whole‐heart isotropic‐resolution motion‐compensated joint T1/T2 mapping and water/fat imaging. Magn Reson Med, 84(6), 3009-3026.
[4] Christodoulou, A. G., Shaw, J. L., Nguyen, C., Yang, Q., Xie, Y., Wang, N., & Li, D. (2018). Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature biomedical engineering, 2(4), 215-226.
[5] Serry, F. M., Ma, S., Li, D., & Christodoulou A. G. (2020) “Dual Flip-angle IR-FLASH for B1+ Insensitive T1 Mapping: Application to T1 CMR Multitasking.” In Proceedings of International Society of Magnetic Resonance in Medicine (ISMRM), 28th Annual International Conference, 2020.
[6] Mao, X., Serry, F. M., Ma, S., Hu, Z., Han, F., Xie, Y., L, D., & Christodoulou, A. G., “3D Free-Breathing, Non-ECG, T1-T2-B1+ Cine Mapping with Cardiac MR Multitasking,” in Proceedings of Society of Magnetic Resonance Angiography (SMRA), 32nd Annual International Conference, 2020.
[7] Mao, X., Serry, F. M., Cokic, I., Ma, S., Hu, Z., Han, F., Lee., H., Xie, Y., L, D., & Christodoulou, A. G., “3D Free-Breathing, Non-ECG, T1-T2-B1+ Cine Mapping with Cardiac MR Multitasking,” submitted to Society for Cardiovascular Magnetic Resonance (SCMR), 24th Annual International Conference, 2021.
[8] Weingärtner, S., Meßner, N. M., Budjan, J., Loßnitzer, D., Mattler, U., Papavassiliu, T., ... & Schad, L. R. (2017). Myocardial T 1-mapping at 3T using saturation-recovery: reference values, precision and comparison with MOLLI. Journal of Cardiovascular Magnetic Resonance, 18(1), 84.
Figures