0688
Single-shot model-based non-rigid motion-corrected T1 rho mapping for endogenous assessment of myocardial injury1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux, INSERM, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Siemens Healthcare France, Saint-Denis, France, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 6Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 7Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France
Synopsis
Magnetic resonance T1 rho mapping may detect myocardial injuries without the need for exogenous contrast agents. However, multiple and differently T1 rho weighted co-registered acquisitions are required, and the lack of robust motion correction limits its clinical translation. This study introduces a novel automated model-based non-rigid motion correction technique for myocardial T1 rho mapping that makes use of the known signal model to drive the motion correction process. The performance, efficiency and clinical feasibility of the developed framework was investigated prospectively in a cohort of 30 patients with a broad range of ischemic and non-ischemic cardiomyopathies.
INTRODUCTION
Magnetic resonance T1 rho (T1ρ) mapping may detect myocardial injuries without exogenous contrast agents.1 However, multiple co-registered acquisitions are required, and the lack of robust motion correction limits its clinical translation. This study introduces a novel automated model-based non-rigid motion correction technique for myocardial T1ρ mapping that makes use of the known signal model to drive the motion correction process. The performance, efficiency and clinical feasibility of the developed framework was investigated prospectively in a cohort of 30 patients with a broad range of ischemic and non-ischemic cardiomyopathies.METHODS
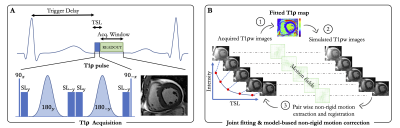
Acquisition: A single-shot electrocardiogram-triggered bSSFP 2D adiabatic T1ρ mapping prototype sequence collecting five T1ρ-weighted (T1ρw) images with different spin lock times (TSL=[0,10,20,35,50]ms) within a single breath-hold was implemented on a 1.5T clinical scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). The T1ρ module (Figure 1A) first plays out a 90º tip-down pulse along the x-axis to rotate the magnetization, followed by 4 spin-lock pulses with alternating phases ($$$SL_{\pm y}$$$) and fixed duration, and two adiabatic refocusing pulses ($$$180_{\pm y}$$$). An additional 90º tip-up pulse is played out to return the magnetization to the z-axis resulting in the cluster ($$$90_x - SL_y - 180_y - SL_{-y} - SL_y - 180_{-y} - SL_{-y} - 90_{-x}$$$).Adiabatic pulses were employed for their reduced sensitivity to a broad range of B0 and B1 field inhomogeneities.2 The amplitude of the spin-lock RF pulse was set to 500Hz.
Reconstruction: To address the problem of residual respiratory motion, a unified optimization framework consisting of a joint T1ρ fitting and model-based non-rigid motion correction algorithm, insensitive to contrast change, was implemented inline for fast (~30s) and direct visualization of the T1ρ maps. The proposed respiratory motion correction framework is shown in Figure 1B and can be formulated as the following optimization problem:
$$( \theta^\star , p^\star ) = \underset{\theta , p}{\mathrm{argmin}} \sum_{t=1}^5 \Vert f_t (p) - E_{\theta_t}y \Vert_2^2 + \mu\Vert Gp \Vert_2^2 + \lambda S(\theta_t) $$
Where $$$y$$$ are the acquired multi-contrast single-shot images, $$$ p = \begin{pmatrix} T1ρ \\ M_0 \end{pmatrix} $$$ are the unknown parameters to recover, with $$$M_0$$$ being the initial longitudinal magnetization, T1ρ the final map, and $$$f_t(p)=M_0 \exp(-\frac{TSL_t}{T1ρ})$$$ our two-parameter fitting model. The warping operator describes a non-rigid deformation $$$\theta_t$$$ for each image $$$t$$$. Because non-rigid motion field estimation is an ill-posed inverse problem, a regularization term that penalizes the L2-norm of motion-field gradients was employed ($$$S(\theta_t) = \Vert \nabla \theta_t \Vert_2^2$$$). Furthermore, an additional spatial smoothness constraint $$$G$$$, returning the spatial gradients of each parameter map, was added to the parameter map to reduce local variations and make the technique more robust to noise. The two scalars $$$\mu$$$ and $$$\lambda$$$ denote the regularization weights and were empirically set to 0.01 and 8e-3, respectively. The optimization is solved using variable splitting.
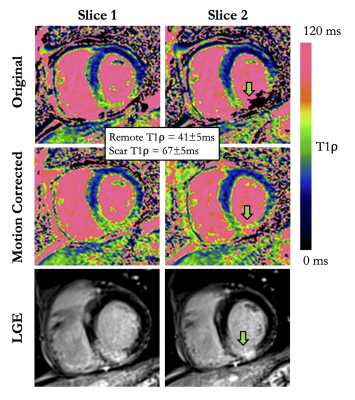
Imaging & Analysis: The technique was tested in 30 patients with suspected myocardial injury. Scan parameters were: in-plane resolution=1.4x1.4mm2, field-of-view=190x220mm2, slice thickness=8mm, RF excitation angle=70º, TE/TR=1.2/2.7ms, phase partial Fourier=6/8, 72 segments, GRAPPA x2. Acquisition was done in mid-diastole during 13 heartbeats (repetition time of 3 heartbeats to allow for full magnetization recovery) in a single breath-hold. Three short-axis slices were acquired (basal, mid, and apical). To quantify motion and assess the performance of motion correction, epicardial and endocardial myocardial contours were drawn on each T1ρw images (non-corrected and motion-corrected) using a custom MATLAB software. The Dice similarity coefficient (DSC)3 and maximum perpendicular distance (MPD) were used to quantify motion and evaluate motion correction. The MPD calculates the maximum displacement of endocardial and epicardial contours across the T1ρw images. The quality of T1ρ maps was scored by an expert radiologist (1-nondiagnostic with severe motion artifacts, 4-excellent image quality with no motion artifact). T1ρ mapping was then compared to cine imaging, T2 mapping and conventional post-contrast 2D late gadolinium enhancement (LGE). T1ρ values were assessed in remote and injured areas, using LGE as reference.
RESULTS
Despite breath-holds, cardiac motion throughout T1ρw imaging was large in patients (22.1±13.5mm). Compared with non-corrected images, the model-based non-rigid motion correction improved the alignment of T1ρw images, with higher DSC (87.7±5.3% vs. 82.2±7.5%, P<0.01), and lower MPD (12.8±9.8mm vs. 22.1±13.5mm, P<0.01). Consequently, T1ρ values significantly increased after motion correction (mean remote T1ρ 48.8±6.5ms vs. 45.9±6.3ms, P=0.02), whereas the precision of T1ρ values was similar (T1ρ SD 4.2±1.2ms vs. 4.2±1.3ms, P=0.93). This resulted in significantly improved quality of the T1ρ maps (score 3.6±0.6 vs. 2.1±0.9, P<0.01) that was graded as excellent for 19/30 (63%) of the motion corrected T1ρ maps, and 2/30 (7%) of the non-corrected T1ρ maps. Using this approach, T1ρ mapping identified LGE in patients with 93% sensitivity and 89% specificity. T1ρ values in injured (LGE positive) areas were significantly higher than in the remote myocardium (68.4±7.9ms vs. 48.8±6.5ms, P<0.01). The benefit of correcting for respiratory motion can be appreciated in Figure 2. Representative examples of non-corrected and motion-corrected myocardial T1ρ maps are shown in Figure 3.CONCLUSION
The proposed motion-corrected T1ρ mapping framework shows promise for the quantitative characterization of myocardial injuries with relatively low sensitivity to respiratory motion and field inhomogeneity. With a 40% T1ρ increase in LGE-positive segments, this technique appears to be a robust and contrast-free adjunct to LGE for gaining new insight into myocardial structural disorders.Acknowledgements
No acknowledgement found.References
[1] Witschey et al. In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012;14:1-9 doi: 10.1186/1532-429X-14-37.
[2] Nezafat R et al. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3T. Magn. Reson. Med. 2006;55:858-864 doi: 10.1002/mrm.20835.
[3] Dice LR. Measures of the amount of ecologic association between species. Ecology 1945 doi: 10.2307/1932409.
Figures