0687
Probing Human Myocardial Krebs Cycle Metabolism and Response to Glucose Challenge using Hyperpolarized [2-13C]Pyruvate MR Spectroscopy1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2School of Pharmacy, University of California, San Francisco, San Francisco, CA, United States, 3Department of Medicine-Cardiology, University of California, San Francisco, San Francisco, CA, United States
Synopsis
This study explored the safety and feasibility to visualize real-time myocardial Krebs cycle energetics in healthy volunteers using hyperpolarized [2-13C]pyruvate MR spectroscopy, and to investigate the response to oral glucose challenge. Identified metabolic products included [2-13C]lactate, Krebs cycle-related intermediate [5-13C]glutamate, and [1-13C]acetylcarnitine, a key player in the “carnitine shuttle” of mitochondrial fatty acid oxidation. Upon oral glucose challenge, the levels of all three products increased, illustrating the metabolic flexibility of human heart to switch between fatty acid and carbohydrates.
Purpose
Despite advances in the medical management and research, heart diseases remain the top cause of death both in the US and globally1. There is mounting evidence that shifts in metabolism and substrate selection are present at early stages of heart disease and cardiomyopathies, but it is challenging to non-invasively measure these shifts in vivo2. Previous work has shown feasibility of hyperpolarized [1-13C]pyruvate in the human heart3,4,5 and detection of [1-13C]lactate facilitated by lactate dehydrogenase (LDH) and 13C-bicarbonate facilitated by pyruvate dehydrogenase (PDH) as pyruvate enters the Krebs cycle. The goal of this study was to assess the feasibility of human studies probing myocardial Krebs cycle metabolism using hyperpolarized [2-13C]pyruvate which, in animal models, is able to detect additional Krebs cycle metabolites of glutamate, citrate and acetylcarnitine6,7,8.Methods
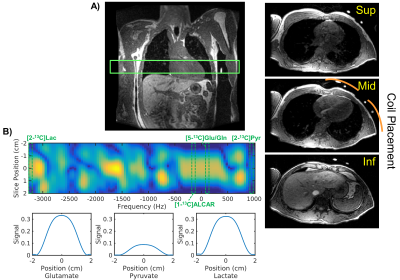
Hyperpolarized-13C Exam: GMP [2-13C]pyruvic acid was prepared and polarized in a 5T GE SpinLab (GE Healthcare, Chicago IL) polarizer for at least 2.5 hours. The dissolution yielded 202-245 mM(mean:228) pyruvate with 27.1-31.2%(mean:28.5) polarization and 0.6-2.2uM residual trityl radical. Two healthy volunteers (male, age:40-42 y/o, median:41) were enrolled in this study and received two injections of [2-13C]pyruvate. The subjects were on a > 4 hour fast at the baseline hyperpolarized scan. Another hyperpolarized scan was acquired 30 minutes after p.o. glucose challenge with sports drink containing approximately 29 grams of sugar. Serum glucose level was measured right before each hyperpolarized injection. The study was conducted on a clinical 3T system (MR750, GE Healthcare) using a clamshell 13C transmitter and an 8-channel paddle receiver array. As illustrated in Figure 1, the two paddles were positioned such that one paddle covers the top and the other the side of the chest wall. The study protocol was approved by the UCSF Institutional Review Board and an FDA IND.Pulse Sequence: A multiband spectral-spatial RF pulse (Figure 1B) was used to excite frequencies for [2-13C]pyruvate, [5-13C]glutamate/[5-13C]glutamine/[1-13C]acetylcarnitine (ALCAR), and [2-13C]lactate with 5°, 20°, and 20°, respectively. Dynamic slab spectroscopy (TE = 3.7ms) was acquired at 3s intervals, targeting a 5cm-thick axial slab over the left ventricle.
Data Processing and Analysis: The spectroscopy data was processed using MATLAB (MathWorks, Natick MA) and Mestrenova (Mestrelab, Spain). Briefly, the array channels were combined using pre-whitened SVD as previously described9. The dynamic spectra were enhanced using SVD rank truncation10 with rank=10, chosen based on the number of distinct 13C species. This was followed by phase and baseline corrections, and peak quantification in Mestrenova.
Results and Discussions
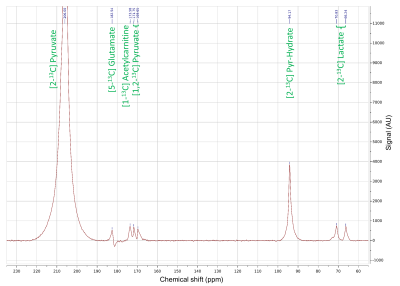
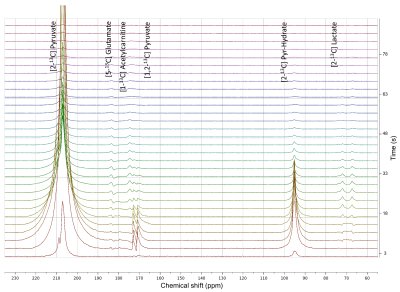
All [2-13C]pyruvate injections were well tolerated without adverse events. The serum glucose levels were 81 and 119(mg/dL) in Volunteer 1 at the time of baseline and post-glucose C2 pyruvate injections, and 76 and 104 in Volunteer 2, respectively.The substrates and products in the spectra (Figure 2) were assigned [5-13C]glutamate(182.5ppm), [1-13C]acetylcarnitine(ALCAR, 173.6ppm), [2-13C]lactate(70.8, 66.2ppm), [1,2-13C]pyruvate(171.8, 169.7ppm), and [2-13C]hydrate(94.2ppm), respectively, referencing to [2-13C]pyruvate (206ppm). The substrate/product assignment was consistent with their kinetic profiles (Figures 3&4). In contrast to a prior work looking at [2-13C]pyruvate in healthy human brain11, ALCAR was notably present in the heart, whereas [5-13C]glutamine/[1-13C]citrate (temporarily assigned, 179.6ppm) was low/absent. The summed apparent SNR of pyruvate, lactate, glutamate, and ALCAR were 1979, 71, 24, and 22 after enhancement, respectively.
The metabolic product-to-pyruvate ratios before and after glucose challenge were summarized in Figure 4B Table. The baseline lactate and ALCAR levels were notably comparable between the two individuals. Also consistent were the percentage changes, with 40.9% increase in lactate/pyruvate, 24.2% in glutamate/pyruvate, and 114% in ALCAR/pyruvate. Interestingly, Volunteer 2 had lower fasting glutamate, and the slight variability in intersubject glutamate % changes are likely attributed to SNR. Figure 4C Table summarized the conversion rate constants, calculated to a first-order approximation using an “input-less” model12. The increase of metabolic products [2-13C]lactate and [5-13C]glutamate upon glucose challenge was consistent with a prior study4 reporting the significant elevation of [1-13C]bicarbonate and slight increase of [1-13C]lactate in a normal volunteer cohort, indicative of the metabolic flexibility of the heart dictated by Randle cycle13.
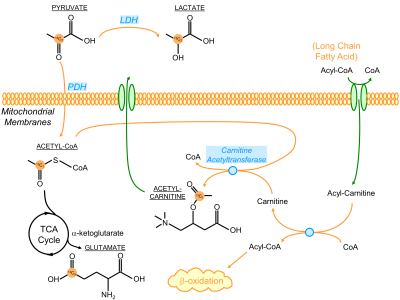
The presence of [1-13C]ALCAR highlights its essential role in the carnitine shuttle as a part of fatty acid β-oxidation, which typically fuels ~70% of the energetics in a healthy heart. An ostensible explanation for the elevation of ALCAR in response to glucose challenge is the increased mitochondrial acetyl-CoA labeling being transferred onto carnitine via carnitine acetyltransferase (Figure 5). However, its real mechanism is likely more complex and remains of great interest for future studies.
In summary, it is safe and feasible to interrogate Krebs cycle energetics in the healthy human heart using hyperpolarized [2-13C]pyruvate. The level of Krebs cycle intermediates and acetylcarnitine labeling increased upon oral glucose challenge. This proof-of-concept [2-13C]pyruvate spectroscopy study of human heart warranted future development of imaging-based acquisition techniques on a dedicated cardiac MR software platform14 and investigation of Krebs cycle modulation in disease processes4,5.
Conclusions
For the first time, hyperpolarized [2-13C]pyruvate MRS was applied for the real-time visualization of Krebs cycle metabolism in healthy human heart. The identification of downstream glutamate and acetylcarnitine was consistent with the Krebs cycle intermediates and fatty acid oxidation in myocardium.Acknowledgements
This work was supported by grants from the UCSF Resource Allocation Program, Myokardia Inc. Myoseeds program, NIH (P41EB013598), and a postdoctoral fellowship from the American Heart Association.References
[1] World Health Organization; https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates
[2] Timm KN et al., Cardiac applications of hyperpolarised magnetic resonance. Prog Nucl Magn Reson Spectrosc 2018; 106, 66-87
[3] Cunningham CH et al., Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circulation Research 2016; 119(11), 1177-1182
[4] Rider, OJ et al., Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circulation Research 2020; 126(6), 106617.
[5] Park, JM et al., Effect of Doxorubicin on Myocardial Bicarbonate Production from Pyruvate Dehydrogenase in Women with Breast Cancer. Circulation Research 2020; 127, 1568-1570.
[6] Schroeder MA et al., Real‐time assessment of Krebs cycle metabolism using hyperpolarized C magnetic resonance spectroscopy. The FASEB journal 2018; 23(8), 2529-2538
[7] Schroeder MA et al., Hyperpolarized 13C magnetic resonance reveals early‐and late‐onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail 2013; 15(2), 130-140
[8] Schroeder MA et al., The cycling of acetyl-coenzyme A through acetylcarnitine buffers cardiac substrate supply: a hyperpolarized 13C magnetic resonance study. Circ Cardiovasc Imaging 2012; 5(2), 201-209
[9] Vareth M et al., A comparison of coil combination strategies in 3D multi‐channel MRSI reconstruction for patients with brain tumors. NMR Biomed 2019; 31(11), e3929
[10] Brender, JR et al., Dynamic Imaging of Glucose and Lactate Metabolism by 13C-MRS without Hyperpolarization. Scientific Reports 2019; 9(1), 3410.
[11] Chung, BT et al., First hyperpolarized [2-13C] pyruvate MR studies of human brain metabolism. J Magn Reson Imaging 2019; 309, 106617.
[12] Larson PEZ et al., Investigation of analysis methods for hyperpolarized 13C‐pyruvate metabolic MRI in prostate cancer patients. NMR Biomed 2018; 31(11), e3997.
[13] Tyler, DJ et al., Demonstrating the Randle cycle in vivo: assessment of physiological alterations in human cardiac metabolism using hyperpolarised 13C MR spectroscopy. Proceedings of the ISMRM, 2017
[14] Tang, S et al., A regional bolus tracking and real‐time B1 calibration method for hyperpolarized 13C MRI. Magn Reson Med 2019; 81(2), 839-851.
Figures