0686
Feasibility of creatine chemical exchange saturation transfer (CEST) imaging in evaluating cardiac dysfunction in acute infarct heart1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2United Imaging Healthcare America, Houston, TX, United States, 3Cardiology Division, West China Hospital, Sichuan University, Chengdu, China
Synopsis
This study aims to investigate the feasibility of creatine (Cr) CEST imaging in assessing cardiac contractile function impairment. Eleven MI pigs underwent cine, Cr CEST and LGE imaging at 3T. Significant reduction of Cr CEST and function indices (i.e., CS, RS, WT and WM) was shown in infarct myocardium compared to that in the remote region. Cardiac function indices were shown to decrease with Cr CEST signal with moderate correlations (P<0.001). The study demonstrated the intrinsic linkage between creatine metabolic and functional changes in MI heart, suggesting the feasibility of Cr CEST in evaluating cardiac dysfunction at the molecular level.
Introduction
Creatine (Cr) metabolism plays a critical role in maintaining normal cardiac function. Chemical exchange saturation transfer (CEST), capable of probing chemical exchange between amine protons on Cr and protons in bulk water, provides a novel way to investigate heart creatine metabolism in normal and pathological states [1-3]. This study aims to investigate the feasibility of Cr CEST in estimating contractile activity degradation in acute infarct hearts.Materials and methods
MRI study: The study was approved by the local Institutional Animal Care and Use Committee. Eleven adult Bama pigs were induced MI by permanently ligating LAD coronary artery distal to the first diagonal branch. Three days later, the animals underwent MRI studies on a 3T scanner (uMR 790, Shanghai United Imaging Healthcare, Shanghai, China). Cardiac quiescent period was identified from cine imaging (0.95×0.95×8.0 mm3, TR/TE=3.1/1.5 ms, 25 retrospectively reconstructed cardiac phases, flip angle=50°). Then, ECG-triggered and respiration-navigated single-slice CEST scans were performed at the short-axis plane covering infarction (1.1×1.1×8.0 mm3, matrix size=270×288, TE=1.2 ms, flip angle=50°, iPAT=2). Six Gaussian-shaped saturation pulses with equivalent B1 of 3.8 μT were applied in the CEST preparation module (pulse duration=40 ms, inter-pulse delay=40 ms, flip angle=2700°). Data was acquired using a GRE-bSSFP readout at cardiac quiescent period. In addition to a reference scan without RF irradiation, 31 CEST-weighted images were collected with saturation frequency offsets equally distributed between ± 4.5 ppm. Finally, LGE imaging was completed 5-10 minutes after the contrast agent injection (0.1 mmol/kg, gadopentetate, Kangchen, Guangzhou, China) using a phase sensitive inversion recovery technique (0.63×0.63×8.0 mm3, TR/TE/TI=4.37/1.78/350 ms, flip angle=25°).Data analysis: From cines, heart contractile function, including wall motion (WM), wall thickening (WT) , circumferential strain (CS), and radial strain (RS), were calculated using Medis Suite 3.2 (Medis Medical Imaging Systems, Leiden, Netherlands) and averaged between two independent observers. Meanwhile, CEST data was processed using custom-written MATLAB codes. The algorithm of minimizing residual complexity [4] was employed to co-register CEST-weighted images and manual adjustment was conducted if necessary. Pixel-wised Z-spectrum (MZ) was normalized by the signal without RF irradiation (M0), interpolated by smoothing splines, and centered to the water resonance [5]. Then, the normalized Z-spectrum was fitted using a probabilistic combination of three-pool Lorentzian functions [1] to resolve saturation transfer effects of magnetization transfer (MT), direct water saturation (DWS) and Cr pool with their chemical shifts at -1.5, 0 and 1.8 ppm, respectively. The amplitude of the Cr pool is defined as the Cr CEST. Myocardium wall was divided into six equiangular segments. Anterior/anteroseptal myocardium, typically induced infarction by LAD ligation, was therefore defined as infarct region, and their adjacent segments (i.e., anterolateral and inferolateral myocardium) and the remaining segments (i.e., inferior and inferoseptal myocardium) as adjacent and remote regions, respectively. Cr CEST and cardiac function indices of CS, RS, WT and WM were measured in each segment. One-way ANOVA was applied with P<0.05 as significant. Pearson correlation was conducted between segmental based Cr CEST and cardiac function indices. Data are presented as mean ± SD.
Results and discussion
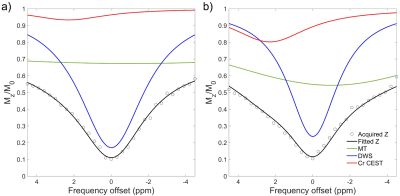
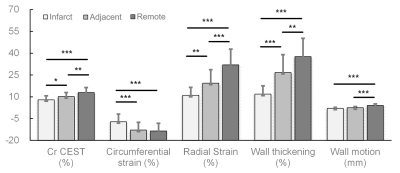
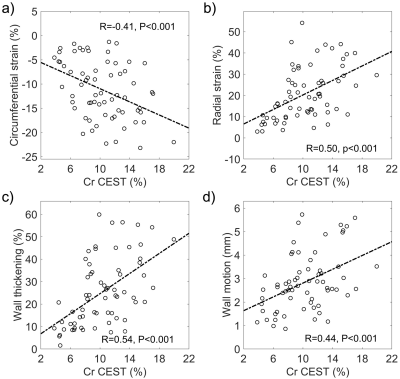
Figure 1 illustrates representative Z-spectra in infarct and remote myocardium. The amplitude of the resolved CEST curve in infarct tissue was found to substantially smaller than that in the remote myocardium. Infarct myocardium identified as hyperintense area on T1w LGE image (Fig. 2b) exhibited substantially lower Cr CEST contrast (Fig. 2a). Cardiac regional function indices (e.g., CS, RS, WT and WM) were determined from ED (Fig. 2c) and ES cines (Fig. 2d), in six equiangular segments. Noticeable contractile dysfunction was shown in regions with or close to infarction as compared to remote areas (Fig. 2e-h). Cr CEST, RS and WT were found to significantly decrease from remote, adjacent to infarct myocardium (Fig. 3, P<0.05). In addition, the absolute value of CS in infarct regions was significantly smaller than that in the other two regions (P<0.001). Meanwhile, remote myocardium showed significantly greater wall motion compared to that in infarct and adjacent regions (P<0.001). Furthermore, absolute values of circumferential and radial strains were found to increase with Cr CEST (Fig. 4), showing moderate correlations with R = 0.41 (P<0.001) and 0.50 (P<0.001) for CS and RS, respectively. Meanwhile, both WT and WM exhibited positive correlation with Cr CEST, and the respective correlation coefficients were 0.54 (P<0.001) and 0.44 (P<0.001).Conclusion
The study demonstrated the close linkage between creatine metabolic and contractile changes in MI heart. Capable of reflecting creatine metabolism that alters earlier than functional degradation, Cr CEST has great potential to be used a sensitive imaging biomarker for the evaluation of myocardium contractile impairment at the molecular level.Acknowledgements
National Natural Science Foundation of China (81571668, 81871348 and 91859102) and Guangdong Special Support Program (2016TQ03R272).References
[1] Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med 2014;20(2):209-214.
[2] Pumphrey A, Yang Z, Ye S, Powell DK, Thalman S, Watt DS, Abdel-Latif A, Unrine J, Thompson K, Fornwalt B, Ferrauto G, Vandsburger M. Advanced cardiac chemical exchange saturation transfer (cardioCEST) MRI for in vivo cell tracking and metabolic imaging. NMR Biomed 2016;29(1):74-83.
[3] Zhou Z, Nguyen C, Chen Y, Shaw JL, Deng Z, Xie Y, Dawkins J, Marban E, Li D. Optimized CEST cardiovascular magnetic resonance for assessment of metabolic activity in the heart. J Cardiovasc Magn Reson 2017;19(1):95.
[4] Myronenko A, Song X. Intensity-Based Image Registration by Minimizing Residual Complexity. IEEE Transactions on Medical Imaging 2010;29(11):1882-1891.
[5] Stancanello J, Terreno E, Castelli DD, Cabella C, Uggeri F, Aime S. Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast Media Mol Imaging 2008;3(4):136-149.
Figures