0679
Hyperpolarized δ-[1-13C]gluconolactone detects response to chemotherapy in brain tumors in vivo1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States
Synopsis
Glucose metabolism via the pentose phosphate pathway (PPP) is typically upregulated in tumors, including gliomas. We previously showed that hyperpolarized δ-[1-13C]gluconolactone metabolism via the PPP to 6-phospho-[1-13C]gluconate (6PG) differentiates tumor from contralateral normal brain in preclinical glioma models. Here, we examined the ability of hyperpolarized δ-[1-13C]gluconolactone to probe response to temozolomide, which is a key chemotherapeutic drug for glioma patients. Our studies in live cells and rats bearing orthotopic gliomas indicate that 6PG production from hyperpolarized δ-[1-13C]gluconolactone serves as an early biomarker of response to temozolomide, a finding that has the potential to improve treatment response monitoring for glioma patients.
INTRODUCTION
The pentose phosphate pathway (PPP) generates NADPH and ribose 5-phosphate, which play a role in scavenging reactive oxygen species and in the nucleotide biosynthesis. As such, the PPP is typically upregulated in cancer cells, including glioma cells, to address the redox and biosynthetic needs associated with rapid cell proliferation [1,2]. The identification of magnetic resonance spectroscopy (MRS)-based metabolic biomarkers of PPP flux will enable non-invasive tumor imaging. Hyperpolarized δ-[1-13C]gluconolactone enters the cell via glucose transporters and is trapped by phosphorylation to 6-phospho-δ-[1-13C]gluconolactone. Subsequent metabolism to 6-phospho-[1-13C]gluconate (6PG) via the PPP was first demonstrated ex vivo in isolated perfused liver [3]. We recently established that hyperpolarized δ-[1-13C]gluconolactone flux to 6PG, which is elevated in tumor vs. contralateral normal brain, serves to non-invasively assess tumor burden in rats bearing orthotopic glioma xenografts in vivo [4,5]. The goal of the current study was to assess the utility of hyperpolarized δ-[1-13C]gluconolactone for assessment of glioma response to therapy in vivo. Chemotherapy with temozolomide (TMZ) is standard of care for glioma patients [6,7]. Here, we show that hyperpolarized δ-[1-13C]gluconolactone flux to 6PG is reduced following TMZ treatment in live cell suspensions. Importantly, 2D echo planar spectroscopic imaging (EPSI) studies point to reduced 6PG production from hyperpolarized δ-[1-13C]gluconolactone as a potential biomarker of response to TMZ in rats bearing orthotopic gliomas in vivo.METHODS
Cell models: We performed studies on three glioma models, the standard U87 model as well as the patient-derived GS2 and BT88 models [8,9]. U87 and GS2 cells were maintained as monolayers in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2mM glutamine and 100U/ml penicillin and streptomycin. BT88 cells were grown as neurospheres in serum-free medium as described [9].δ-[1-13C]gluconolactone probe preparation: δ-[1-13C]gluconolactone was synthesized and polarized as previously described [3]. Briefly, 2M δ-[1-13C]gluconolactone was dissolved in 3:1 water:glycerol and mixed with 15mM trityl radical OX063. Once polarization was achieved (~1.5h), the sample was dissolved in phosphate-buffered saline (pH~7) to a final concentration of 8mM for cell studies and 37mM for in vivo studies.
Temozolomide treatment: For cell studies, U87 and GS2 cells were treated with 100μM TMZ or DMSO (vehicle) for 72h. For in vivo studies, rats bearing orthotopic BT88 tumors were intraperitoneally treated with 50mg/kg TMZ once a day.
Hyperpolarized 13C-MRS in live cells: Hyperpolarized δ-[1-13C]gluconolactone, prepared as described above, was injected into live cells (~3x107) in a 5mm NMR tube and 13C-MRS spectra acquired every 3s for 300s on a Varian 500MHz NMR spectrometer using a 13° pulse. Signal-to-noise (SNR) ratios were quantified using MestReNova.
Hyperpolarized 13C-MRS in vivo: All MR studies were performed on a horizontal 3T scanner (BioSpec 105mm bore diameter, Bruker) equipped with a dual-tuned 1H-13C volume coil. Male athymic nu/nu rats were intracranially injected with 3x105 BT88 cells [10]. Tumor growth was assessed by axial T2-weighted MRI acquired using a spin echo (TurboRARE) sequence. Following injection of 2.2ml of hyperpolarized δ-[1-13C]gluconolactone (prepared as described above) via a tail-vein catheter over 15s, 2D EPSI data was acquired using a spectral spatial (SPSP) sequence [4,5] with a spatial resolution of 5.375x5.375x8mm3 (TR=3s/NR=20), 15.2° pulse on 6PG and 3.4° pulse on δ-[1-13C]gluconolactone. 13C spectra were analyzed by calculating the area under the δ-[1-13C]gluconolactone and 6PG peaks. Intensity heat maps were produced by interpolating the data using a Lanczos-2 kernel. The SNR of the substrate, as well the product to substrate ratios were evaluated using in-house Matlab code [10].
Statistical analysis: All results are expressed as mean±STD. Statistical significance was assessed using an unpaired two-tailed Student’s t-test with p<0.05 considered significant.
RESULTS AND DISCUSSION:
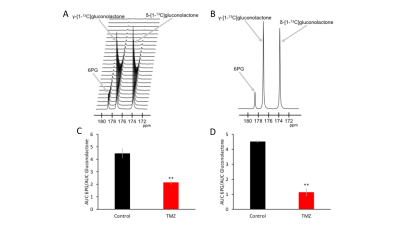
First, we examined the effect of TMZ on hyperpolarized δ-[1-13C]gluconolactone flux to 6PG in live U87 and GS2 cells. A representative 13C spectral array and summed 13C spectra from vehicle-treated U87 cells are shown in Fig.1A-1B. 6PG production from hyperpolarized δ-[1-13C]gluconolactone was significantly reduced following TMZ treatment in both U87 (52% drop, N=3; p=0.006; Fig.1C) and GS2 (75% drop, N=3, p=0.002; Fig.1D) models.We then performed 2D EPSI studies in rats bearing orthotopic BT88 tumors treated with TMZ and examined the ability of hyperpolarized δ-[1-13C]gluconolactone to assess response to TMZ. In line with previous results [4,5], examination of metabolic heatmaps prior to treatment with TMZ (Fig. 2A-2C) confirmed that ratio of 6PG to δ-[1-13C]gluconolactone was higher in the tumor compared to contralateral normal brain. Metabolic heatmaps of the 6PG/δ-[1-13C]gluconolactone ratio generated 48h after TMZ treatment showed a drop relative to pre-treatment heatmaps (compare Fig. 2C & 2F). In contrast, there was no change in the SNR of δ-[1-13C]gluconolactone (see Fig. 2B & 2E). Importantly, there was no change in tumor volume (Fig. 2A & 2D; pre-treatment = 105mm3, post-treatment = 130mm3), suggesting that 6PG production from hyperpolarized δ-[1-13C]gluconolactone can serve as an early metabolic biomarker of response to TMZ in gliomas in vivo.
CONCLUSIONS
Our studies with live cells and preclinical rodent models suggest that hyperpolarized δ-[1-13C]gluconolactone can be used to assess glioma response to chemotherapy with TMZ. Importantly, 6PG production from hyperpolarized δ-[1-13C]gluconolactone appears to be an early biomarker of treatment response, prior to volumetric alterations, pointing to its potential to assess pseudoprogression, which is a major challenge in glioma imaging.Acknowledgements
This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315, UCSF Brain Tumor Center SPORE Career Enhancement Program Award (NIH P50CA97257), NIH R01CA172845, NIH R01CA197254, NIH P01CA118816, UCSF LOGLIO collective, NICO and NIH Center grant P41EB013598.References
[1] Dong MA et al, Review of metabolic pathways activated in cancer cells as determined through isotopic labeling and network analysis, Metabolic Engineering (2017) 43:113-124
[2] Patra KC et al, The pentose phosphate pathway and cancer, Trends in Biochemical Sciences (2014) 39:347-54
[3] Moreno KX et al, Hyperpolarized δ‐[1‐13C]gluconolactone as a probe of the pentose phosphate pathway, NMR in Biomedicine 30 (2017) 30(6)
[4] Batsios G et al, In vivo evaluation of pentose phosphate pathway activity in orthotopic glioma using hyperpolarized δ-[1-13C]gluconolactone, ISMRM 2019 #2304
[5] Batsios G et al, Hyperpolarized δ-[1-13C]gluconolactone monitors TERT-induced elevation in pentose phosphate pathway flux in brain tumors in vivo, ISMRM 2020 #129
[6] Choi S et al, Temozolomide-associated hypermutation in gliomas, Neuro Oncol (2018) Sep 3;20(10):1300-1309
[7] Weller M et al, Temozolomide: a milestone in the pharmacotherapy of brain tumors, Future Oncol (2005) Dec;1(6):747-54
[8] Hashizume R et al, A human brainstem glioma xenograft model enabled for bioluminescence imaging, J Neurooncol. (2010) 96(2): 151–159
[9] Kelly J et al, Oligodendroglioma cell lines containing t(1;19)(q10;p10), Neuro Oncol (2010) 12 (7): 745–755
[10] Batsios G et al, In vivo detection of γ-glutamyl-transferase up-regulation in glioma using hyperpolarized γ-glutamyl-[1-13C]glycine, Scientific reports (2020) 10(1):6244
Figures