0678
Hyperpolarized 13C MRI Detects In-Vivo Effect of Exercise on Pyruvate Metabolism in Human Skeletal Muscle1AIRC, UT Southwestern Medical Center, Dallas, TX, United States, 2The Developing Brain Institute, Children’s National Hospital, Washington, DC, United States, 3GE, Chicago, IL, United States, 4Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 5Biochemistry and Molecular Medicine, UC Davis, Davis, CA, United States, 6Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 7Electrical and Computer Engineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Pyruvate dehydrogenase (PDH) and lactate dehydrogenase (LDH) are essential for ATP production in skeletal muscle. However, directly PDH flux in exercising human muscle has been challenging and never assessed. This study was to demonstrate the feasibility of assessing PDH activation and changes in pyruvate metabolism in human skeletal muscle after the onset of exercise using hyperpolarized [1-13C]pyruvate. During moderate flexion-extension exercise, total HP 13C signals (tC), [1-13C]lactate/tC, and [13C]bicarbonate/tC increased significantly compared to resting state. This study demonstrates that PDH flux in skeletal muscle increases rapidly after the onset of exercise and decreases during recovery.
INTRODUCTION
A continuous supply of ATP is required for contracting skeletal muscle. ATP must be generated by glucose metabolism to lactate or oxidative phosphorylation. PDH dictates the fraction of energy derived from glycogen/glucose and fatty acids. The early effect of exercise on PDH flux in human muscle is unknown. PDH activity in skeletal muscle is disrupted in rare congenital defects of the PDH complex and other mitochondrial myopathies. ATP demand and flux through energy-generating pathways is very low in skeletal muscle at rest(1). During modest exercise, glycogen oxidation and PDH flux increase dramatically. PDH contributes to virtually all energy production. HP studies have followed the rapid activation of PDH in rodent muscle using a pyruvate dehydrogenase kinase inhibitor, dichloroacetate(2), but applications in human skeletal muscle have not been explored. This study demonstrates that production of [13C]bicarbonate and [1-13C]lactate from HP [1-13C]pyruvate in human skeletal muscle is sensitive to brief exercise.MATERIALS and METHODS
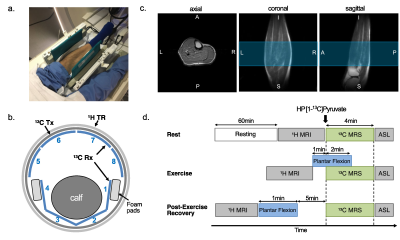
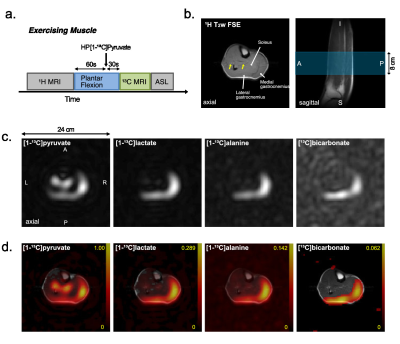
The protocol of this prospective study was approved by the local Institutional Review Board (STU-2018-0227). Informed written consent was obtained from all participants (n = 9, age 42.3 ± 17.5 n = xx, age = xx +/- xx, 6 male and 3 female), who were sedentary adults in good general health with no history of peripheral vascular, systemic, cardiac or musculoskeletal disease. All participants rested for one hour prior to the study and moved to the scanner via wheelchair. The subject was positioned supine with one leg in a 13C/1H dual-frequency RF coil for imaging the calf muscle (Fig.1a-c)(3). After resting MR exams, participants performed plantar flexion exercise (Fig.1d). participants were imaged using a 13C/1H integrative MR protocol, which consists of series of 1H MRI, including arterial spin labeling (ASL), and up to two injections of HP [1-13C]pyruvate at rest, during exercise, or 5-min after exercise (recovery). For 13C MRS, a time-resolved free-induction-decay data were acquired using a slice-selective pulse-and-acquire sequence. For 13C MRI, a metabolite-selective multi-echo spiral imaging sequence was used to image [13C]bicarbonate, [1-13C]lactate, [1-13C]pyruvate, and [1-13C]alanine in an interleaved manner using a spectral-spatial RF pulse that selectively excites the labeled metabolites(4).RESULTS
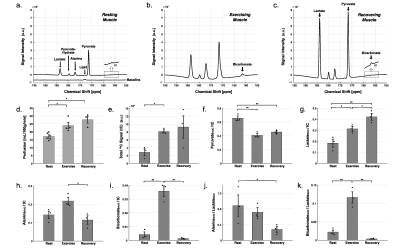
Typical time-averaged 13C data are shown in Figure 2. Perfusion increased markedly after the exercises, from resting-state (17.1 ± 0.9 mL/100g/min), to 24.0 ± 2.1 (P = 0.02) with exercise and 27.6 ± 2.4 (P = 0.03) during recovery. Total HP 13C signal, increased to 2.8-fold with exercise (P = 0.005) and to 3.2-fold during recovery (P = 0.10). Total [1-13C]pyruvate signal, normalized by tC, at rest was significantly larger than during exercise (P = 0.003) or recovery (P = 0.005). Normalized [1-13C]lactate signal was larger during recovery period than at rest (P = 0.008) and during exercise (P = 0.04). Normalized alanine production was largest during exercise as compared to recovering (P = 0.028) or resting (P = 0.06) muscle. Bicarbonate production was significantly larger with exercise than those at rest (P = 0.002) and recovery (P = 0.001). To confirm the source of HP 13C signals, two-dimensional images of calf muscle were acquired from two healthy participants during exercise as shown in Figure 3. To suppress the motion artifacts, the participants were instructed to stop the plantar flexion exercise three seconds before the scan started. From both participants, [1-13C]Pyruvate, [1-13C]lactate, [1-13C]alanine, and [13C]bicarbonate maps were detected with the exercise, and the HP signals were primarily from medial and lateral gastrocnemius. At rest, the product images were at the noise level.DISCUSSION
The exam was well-tolerated, and the results were very sensitive to the exercise state. Using in-magnet plantar flexion exercise, real-time PDH and LDH activities could be monitored. As a fraction of total carbon signal, both protocols were associated with an increase in HP [1-13C]lactate. The presence of bicarbonate during exercise directly indicates activation of PDH, and the disappearance of bicarbonate after exercise suggests rapid deactivation of PDH during the recovery period. The decreased post-exercise HP [1-13C]alanine level relative to the [1-13C]lactate suggests the utility of alanine as an index of intracellular delivery of pyruvate in skeletal muscle. Such results have practical applications for conditions, which may result in muscle ischemia or excessive anaerobic metabolism, such as peripheral vascular (arterial) disease, and myopathy conditions. In future, these imaging markers could be employed in the above conditions for early non-invasive diagnosis and follow-up after treatment.CONCLUSION
The experiments measured pyruvate metabolism to lactate, alanine, and acetyl-CoA of skeletal muscle in humans using HP [1-13C]pyruvate. As compared to the resting-state, calf exercise induced increased perfusion and pyruvate-to-lactate conversion. Essentially no bicarbonate production was detected at rest and minutes after plantar flexion exercise. On the other hand, bicarbonate production was significantly increased during exercise, directly demonstrating flux through PDH during exercise. The HP approach offers then a novel, unique way to study PDH activation and regulation in human skeletal muscle, which plays a key role muscle pathophysiology. The study has then opened a new frontier for non-invasive assessment of muscle physiology.Acknowledgements
National Institutes of Health of the United States (R01 NS107409, P41 EB015908, S10 RR029119, S10 OD018468); The Welch Foundation (I-2009-20190330); The Texas Institute for Brain Injury and Repair; UT Dallas Collaborative Biomedical Research Award (UTD 1907789).References
1. Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;159:738–12.
2. Park JM, Josan S, Mayer D, et al. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol. 2015;218:3308–3318.
3. Ma J, Hashoian RS, Sun C, et al. Development of 1H/13C RF head coil for hyperpolarized 13C imaging of human brain. Intl Soc Magn Reson Med. Montreal, Canada. 2019. #568.
4. Ma J, Hackett EP, Park JM. In vivo T* mapping of hyperpolarized 13C-labeled metabolites using a metabolite-selective multi-echo spiral imaging sequence. Intl Soc Magn Reson Med. Virtual. 2020. #3051.
Figures