0672
Laser Heating Induced Susceptibility Artifacts Cause Significant Temperature Erros in PRF Shift-based MR Thermometry1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Sinovation Medical, Beijing, China, 3Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Philips Healthcare, Beijing, China, 5Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China
Synopsis
MR-guided laser-interstitial thermal therapy (MRgLITT) is a minimally invasive therapeutic method that has created new options for surgically challenging lesions. Most MRgLITT procedures depend on proton-resonance-frequency (PRF) shift-based MR thermometry. However, it can be hampered by magnetic susceptibility changes generated during laser ablation. In this work, we demonstrate for the first time that laser-heating induced susceptibility changes can lead to significant temperature errors, with ex-vivo (pig muscle and brain tissues), in-vivo (Doberman) and clinical (epilepsy patient) experiments. A new algorithm based on multi-echo GRE instead of the conventional single-echo GRE is also introduce to correct the susceptibility-induced temperature errors.
Introduction
The PRF (proton resonance frequency) shift-based MR thermometry 1-5 can be affected by many potential sources of error 6, and can consequently lead to LITT (laser interstitial thermal therapy) hyperthermia due to incorrect temperature monitoring, resulting in severe complications such as dysphasia 7-10, paraplegia 5, and short-term memory loss 11.In this work, we report for the first time that laser heating can lead to significant magnetic susceptibility changes and is, therefore, a possible source of temperature error. We also introduce the idea of using multi-echo GRE (gradient recalled echo) instead of the conventional single-echo GRE in PRF shift-based thermometry to correct such a problem. Experiments were performed on ex-vivo pig muscle and pig brain tissues, in-vivo Dobermans, and patients with epilepsy.
Materials and Methods
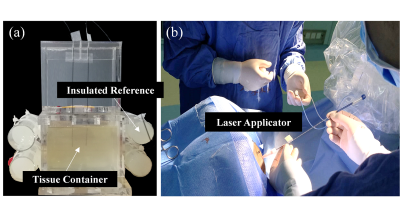
Experiment 1: Different Laser Power LevelsThe ex-vivo pig muscle tissues were placed in a plastic container (as shown in Fig. 1a), and heated at 3 different laser power levels (5W, 8W, and 10W) via the laser ablation system (MRI-Guided Laser Ablation System, Sinovation Medical, China). Heating was stopped once the observation point (5mm away from the optical fiber tip, monitored in real-time) reached 70°C, in order to create comparable heating zones at different heating powers.
Temperature data were acquired on a 3T MRI scanner (Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel receive coil using the GRE sequence: flip angle = 30° , TE =19.3ms, TR = 76ms, matrix = 128×128, FOV = 230× 230 mm2, slice thickness = 5mm, 3 slices parallel to the laser applicator, no gap, GRAPPA = 3, temporal resolution = 3 s/volume.
Experiment 2: Multiple Echoes
The ex-vivo pork and pig brain tissues were heated at 8W for 3 minutes. Two MR-compatible fiber-optic temperature probes were also inserted into the phantoms to measure the ground truth values. The in-vivo Doberman canines were anesthetized and ablated at 8W for 50 seconds.
Temperature data were acquired on a 3T MRI scanner (Ingenia CX, Philips Healthcare, Best, The Netherlands) using the multi-echo flyback GRE sequence: flip angle = 30° , TE = 4/9/14/19ms, TR = 22ms, matrix = 176×176, FOV = 200×200 mm2, no parallel imaging, temporal resolution = 3s/volume. A 16-channel receive coil was used.
Experiment 3: Patients with Epilepsy
Epilepsy patients underwent MRgLITT with a treatment dose at 8W. MRTI (thermal imaging) was acquired (3T, Verio, Siemens Healthcare, Erlangen, Germany) with a multi-echo flyback GRE sequence: TE = 3.9/11.6/19.3ms. Other acquisition parameters were identical to Experiment 1. An 8-channel receive coil was used. Post-treatment T1-weighted gadolinium (Gd) enhanced images were also acquired to visualize the ablation zone.
Temperature Imaging Algorithm
A multi-echo GRE-based algorithm was applied, which gained many advantages from the multi-echo integrated information: (1) Susceptibility-induced temperature errors could be corrected by the shortest TE; (2) Robust phase-unwrapping could be achieved based on the naturally unwrapped phase map of the shortest TE; (3) CSF flow artifacts could be suppressed by a multi-echo linear least-square fit (because motion-induced phase errors did not change with TE). Details were not included due to limited space.
For comparison, a traditional PRF-shift based algorithm 12 was also performed on the single-echo data of Experiment 1-3 acquired either by single-echo or multi-echo GRE.
Results and Dissusion
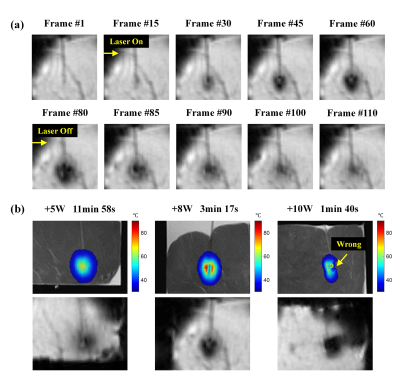
Result 1: Different Laser Power LevelsAs shown in Fig. 2a, laser heating causes a rapid magnitude decrease in the ablation center during LITT treatment. Such signal loss recovers gradually after heating is stopped, indicating the signal loss is not relevant to other reasons but caused by the laser heating itself.
The signal loss and corresponding temperature errors become more apparent (Fig. 2b) with the increase of laser power, despite the shortened heating time. It indicates the susceptibility artifacts are greatly related to the level of heating power.
Result 2: Multi-echoes
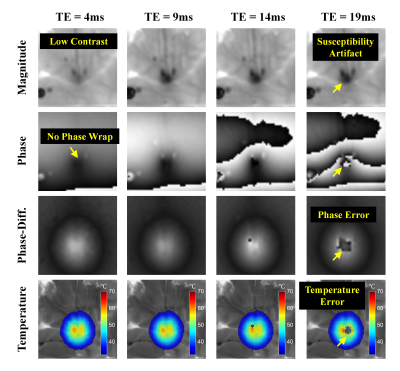
As shown in Fig. 3, the heating center shows a more severe magnitude decrease on longer TEs (longer time for intra-voxel T2* dephasing) and consequently lead to unacceptable errors on the phase-difference and temperature maps. The magnitude of the shortest TE is, on the other hand, almost unaffected by the susceptibility changes.
The mean RMSE (root mean square error) calculated between the multi-echo GRE-measured temperatures and the thermometer-measured temperatures are 0.31°C and 0.52°C in ex-vivo pork and pig brain tissues, respectively.
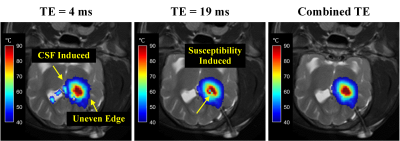
Fig. 4 shows the multi-echo combined temperature map in the canine experiment, free of either CSF-induced errors (appeared in TE=4ms) or the susceptibility-induced errors (appeared in TE=19ms).
Result 3: Epilepsy Patients
Two representative cases are presented in Fig. 5, which show significant susceptibility-induced temperature errors during LITT treatment. The multi-echo GRE based algorithm corrects the susceptibility errors greatly, but fails on Frame 136 in Fig. 5b, which shows mistakenly high temperatures and distortions appearing as a dipole filed pattern. This is because the laser heating is so intense that the shortest TE is also corrupted by the susceptibility artifacts.
Conclusion
We have shown experimentally that laser-heating induced susceptibility artifacts can introduce severe temperature errors in PRF shift-based thermometry. The errors can be greatly reduced by the proposed algorithm based on multi-echo GRE. Still, physicians should consider heating target tissues at lower power or lower temperature to reduce the potential of inducing susceptibility artifacts during LITT treatment.Acknowledgements
References
1. Schneider, W.G., H.J. Bernstein, and J.A. Pople, Proton Magnetic Resonance Chemical Shift of Free (Gaseous) and Associated (Liquid) Hydride Molecules. Journal of Chemical Physics, 1958. 28(4): p. 601-607.
2. Lutz, N.W., A.C. Kuesel, and W.E. Hull, A 1H-NMR method for determining temperature in cell culture perfusion systems. Magn Reson Med, 1993. 29(1): p. 113-8.
3. De Poorter, J., Noninvasive MRI thermometry with the proton resonance frequency method: study of susceptibility effects. Magn Reson Med, 1995. 34(3): p. 359-67.
4. De Poorter, J., et al., Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magn Reson Med, 1995. 33(1): p. 74-81.
5. Ishihara, Y., et al., A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med, 1995. 34(6): p. 814-23.
6. H. Odeen and D. L. Parker, "Magnetic resonance thermometry and its biological applications - Physical principles and practical considerations," Prog Nucl Magn Reson Spectrosc, vol. 110, pp. 34-61, Feb 2019.
7. A. Carpentier, R. J. McNichols, R. J. Stafford, J. P. Guichard, D. Reizine, S. Delaloge, E. Vicaut, D. Payen, A. Gowda, and B. George, "Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumors," Lasers in Surgery and Medicine, vol. 43, pp. 943-950, Dec 2011.
8. A. H. Hawasli, S. Bagade, J. S. Shimony, M. Miller-Thomas, and E. C. Leuthardt, "Magnetic Resonance Imaging-Guided Focused Laser Interstitial Thermal Therapy for Intracranial Lesions: Single-Institution Series," Neurosurgery, vol. 73, pp. 1007-1017, Dec 2013.
9. Rahmathulla, G., et al., Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg, 2012. 90(3): p. 192-200.
10. Reimer, P., et al., MR-monitored LITT as a palliative concept in patients with high grade gliomas: Preliminary clinical experience. Jmri-Journal of Magnetic Resonance Imaging, 1998. 8(1): p. 240-244.
11. D. J. Curry, A. Gowda, R. J. McNichols, and A. A. Wilfong, "MR-guided stereotactic laser ablation of epileptogenic foci in children," Epilepsy & Behavior, vol. 24, pp. 408-414, Aug 2012.
12. Sprinkhuizen, S.M., et al., Temperature-induced tissue susceptibility changes lead to significant temperature errors in PRFS-based MR thermometry during thermal interventions. Magn Reson Med, 2010. 64(5): p. 1360-72.
Figures