0667
Improved Fat and Water Depiction in Musculoskeletal MRI by Control of Through-Slice Chemical-Shift Artifacts in 2D Turbo-Spin-Echo Imaging at 7 T1Siemens Healthcare, Zurich, Switzerland, 2Swiss Center for Musculoskeletal Imaging (SCMI), Balgrist Campus, Zurich, Switzerland, 3Radiology, Balgrist University Hospital, Zurich, Switzerland, 4University of Zurich, Zurich, Switzerland, 5Siemens Healthcare, Erlangen, Germany
Synopsis
The large water-fat frequency difference at 7 T renders imaging of the musculoskeletal (MSK) anatomy very challenging. In particular, through-slice chemical-shift artifacts may manifest in state-of-the-art 2D turbo-spin-echo (TSE) images as partial or locally complete fat-signal loss that radiologists are usually not trained to account for from lower field strengths. In this work, we demonstrate the range of possible through-slice artifacts in MSK images and show that matched RF-pulse bandwidths as high as 1500 Hz for the excitation and refocusing RF-pulses are necessary to consistently perform successful, non-fat suppressed MSK imaging at 7 T.
Introduction
State-of-the-art Turbo-Spin-Echo (TSE) sequences commonly employ a selection of unmatched and moderate RF-pulse bandwidths for the excitation and refocusing pulses to suppress “third arm” artifacts1. However, different bandwidths result in varying chemical-shift displacements along the slice-selection direction. Particularly at 7 Tesla (T), this geometrical mismatch between the excitation and refocusing slice profiles of fat may manifest as a relevant fat-signal loss. Additionally, moderate RF-bandwidths can cause significant through-slice chemical-shift displacement resulting in geometric inconsistencies between imaged water and fat slice. In musculoskeletal applications2, these effects can cause false positive findings, such as bone fractures or neoplastic bone-marrow disease3. The objective of this work was to explore and reduce through-slice chemical-shift artifacts in TSE imaging at 7 T.Theory
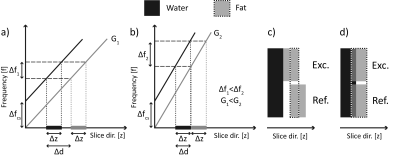
A slice-selective RF-pulse affects fat and water spins in different slices that are separated along the slice-selection dimension by $$$d$$$:$$ d = \Delta{z}\frac{\Delta f_{CS}}{\Delta f}$$ $$$\Delta{z}$$$ is the prescribed slice thickness,$$$\Delta f_{CS} \approx$$$ 1040 Hz @ 7 T is the Larmor-frequency difference between fat and water and $$$\Delta{f}$$$ the RF-pulse bandwidth (Fig. 1).Materials and Methods
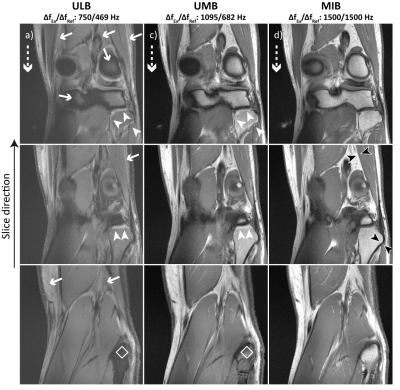
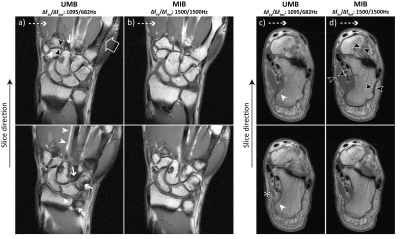
All experiments were performed on a 7 T whole-body MR scanner (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) equipped with a 1Tx/28Rx-channel knee coil (QED, Quality Electrodynamics, Mayfield Village, OH, USA) for knee and foot imaging and a single-channel flex coil (RAPID Biomed, Rimpar, Germany) for wrist imaging. Foot imaging is not the intended use for the knee coil.2D TSE imaging was performed with a standard sequence utilizing (a) unmatched and low bandwidths (“ULB”), (b) unmatched and moderate bandwidths (“UMB”) and (c) a prototype sequence with matched and increased bandwidths (“MIB”). For the ULB, UMB and MIB case, the bandwidths of the excitation/refocusing pulses were set to 750/469 Hz, 1095/682 Hz and 1500/1500 Hz, respectively. Both RF-pulse types had a Hanning-filtered Sinc shape. Apart from the RF-pulse bandwidths, imaging parameters were otherwise identical for each extremity (Fig. 2a).
T1-weighted sagittal knee MRI with UMB and MIB was performed (i) in 10 healthy volunteers (mean age 31 ± 6 years, 6 male). Additional T1-weighted data were acquired from a single subject (34 years, male) (ii) in coronal orientation in the knee (ULB, UMB, MIB), (iii) in coronal orientation in the wrist (UMB, MIB), (iv) in axial orientation in the foot (UMB, MIB). The measurements were conducted in adherence with the federal law and written informed consent was obtained prior to each examination from all subjects.
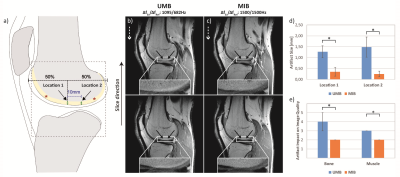
Chemical-shift artifacts in the mostly affected sagittal knee images were measured for UMB and MIB at two standardized locations at the distal femoral bone-cartilage interface (Fig. 3a). The perceived negative impact of through-slice chemical-shift artifacts on image quality in the bone and posterior muscles were graded on a 5-point Likert4 scale (Fig. 2b). Objective and subjective artifact metrics were assessed by two fellowship-trained musculoskeletal radiologists with 15- and 2-years’ experience in musculoskeletal MR imaging. Artifact size and impact on image quality were compared between UMB and MIB using a two-way repeated measures ANOVA and Wilcoxon test, respectively (SPSS, Version 25, IBM Inc, Armonk, NY). The corresponding inter-reader agreement was assessed with the intra-class correlation coefficient (ICC) and Cohen’s Kappa coefficient, respectively. Values for the artifact size and impact on image quality are reported as mean ± standard deviation and median ± interquartile range, respectively. A p-value < 0.05 was considered statistically significant.
Results
Through-slice chemical-shift artifacts at the bone-cartilage interface were statistically significantly smaller with MIB compared to UMB: location 1: 0.35 ± 0.20 mm vs. 1.27 ± 0.27 mm, p < 0.001, location 2: 0.25 ± 0.13 mm vs. 1.48 ± 0.46 mm, p < 0.001, ICC: 0.98. The negative impact of chemical-shift artifacts on image quality was rated statistically significantly smaller with MIB than with UMB: bone: 2 ± 0 vs. 4 ± 1, p < 0.005 (both readers); muscle: 3 ± 0 vs. 2 ± 0, p < 0.005 (both readers), Kappa: 0.69 (Figs. 3 d, e). UMB images displayed multiple artifactual hyperintensities as well as significant fat-signal loss in all examined anatomical regions. MIB images consistently showed high image quality with bright T1-weighted fat-signal, excellent visual resolution of fine tissue structures and improved image sharpness at several bone-muscle, bone-fat and muscle-fat interfaces (Figs. 3 b, c, 4, 5). The specific absorption rate for MIB was approximately doubled compared to UMB.Conclusion
Optimization of RF-pulse bandwidths to address through-slice chemical-shift artifacts allowed consistently successful high-quality concurrent fat- and water TSE imaging at 7 T. Through-slice chemical-shift artifacts as well as their perceived negative impact on image quality were significantly reduced while “third arm” artifacts1 could not be observed in our measurements, using local transmit-receive coils. The proposed sequence optimization has the potential to reduce the likelihood of false readings in musculoskeletal applications at 7 T.Acknowledgements
This research project is partly funded by Balgrist Campus AG, by the University Hospital Balgrist and by Siemens Healthcare AG: CvD, SS and RMH are employees of Siemens Healthcare AG.References
1. Haacke EM, Brown RW, Thompson MR, et al. Magnetic Resonance Imaging: Physical Principles and Sequence Design. 1st ed. New York: John Wiley & Sons; 1999.
2. Juras V, Mlynarik V, Szomolanyi P, et al. Magnetic Resonance Imaging of the Musculoskeletal System at 7T: Morphological Imaging and Beyond. Top. Magn. Reson. Imaging. 2019;28(3):125–135.
3. Singhal V, Bredella MA. Marrow Adipose Tissue Imaging in Humans. Bone. 2019;118:69–76.
4. Jamieson S. Likert scales: How to (ab)use them. Med. Educ. 2004;38(12):1217–1218.
Figures