0663
Radio-pathomic models trained with autopsy tissue samples aligned to MP-MRI predict histopathological features in brain cancer patients.1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 3Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
This study used autopsy tissue in order to develop radio-pathomic models for histopathological features of brain cancer. These models used T1, T1C, FLAIR, and ADC images from 45 patients as input into bagged regression ensembles for cellularity, cytoplasm, and extracellular fluid, using the aligned autopsy tissue samples as ground truth. These models were able to accurately predict these features and were able to find tumor signatures, such as hypercellularity beyond the traditional contrast-enhancing and FLAIR hyperintense regions. These radio-pathomic maps provide new insights into non-invasive signatures of tumor pathology in the post-treatment state and beyond the contrast enhancing region.
Introduction
MR imaging is the central method of monitoring the progression and treatment response of glioblastoma (GBM) and other brain cancers. Pre- and post-contrast T1-weighted imaging monitor disruptions in the blood brain barrier resulting from primary tumor growth and are often used to delineate the primary tumor region 1,2. FLAIR hyperintensities are thought to represent areas of edema resulting from tumor growth 3,4 and areas of restricted diffusion as measured by apparent diffusion coefficient (ADC) maps calculated from diffusion-weighted imaging 5–7. These relationships have typically been pathologically validated via biopsy tissue studies, which may fail to capture information about the tumor growth and proliferation beyond the contrast enhancing region. Additionally, biopsy tissues are often collected prior to radiation treatment and chemotherapy, which may influence the relationships between MRI signatures and the underlying tumor pathophysiology 8,9.This study sought to develop radio-pathomic models of tumor histopathological characteristics using autopsy tissue as ground truth. Specifically, we tested the hypothesis that MRI-based machine learning models trained on autopsy tissue samples are able to accurately predict cell density, cytoplasm density (CYT), and extracellular fluid density (ECF).
Methods
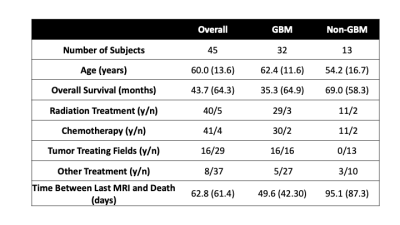
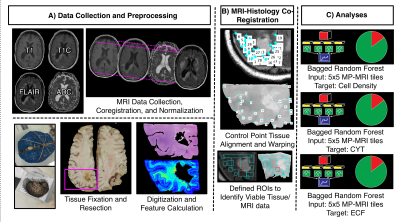
This study included 45 patients with pathologically confirmed brain cancer, whose clinical and demographic characteristics are presented in Table 1. A diagrammatic representation of the data collection process and analysis framework is presented in Figure 1. At autopsy, 93 tissue samples were collected across patients, which were then processed and stained for hematoxylin and eosin (HE). Stained tissue samples were then digitized using a sliding stage microscope and segmented in order to extract cell counts, relative cytoplasm density, and relative extracellular fluid density across each slide. T1, post-contrast T1 (T1C), FLAIR, and ADC images for each patient were collected from each patients’ most recent clinical imaging session prior to death. Each MR image was then aligned to the FLAIR image, and T1, T1C, and FLAIR images were intensity normalized by dividing by the whole brain standard deviation. In-house custom Matlab software was used to align tissue samples to the FLAIR image using a control-point based registration 7,10,11.Bagged regression ensemble algorithms were used to develop the radio-pathomic models for this study. Three models were trained to separately predict voxel-wise cellularity, CYT, and ECF, using 5x5 voxel image tiles from each MR image as inputs. Models were validated using a randomly selected 2/3-1/3 train-test split (train on 30, test on 15). Model performance for each algorithm was quantified using root-mean-squared error (RMSE) values, normalized to the target standard deviation.
Results
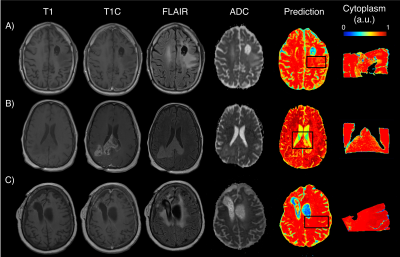
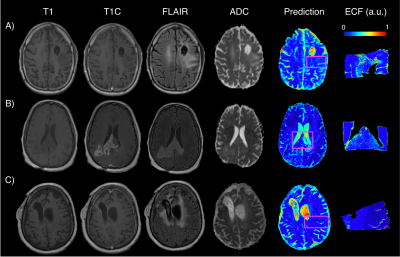
Model performance statistics were as follows: cellularity train RMSE=0.31, test RMSE=0.78; CYT train RMSE=0.32, test RMSE=1.15; ECF train RMSE=0.37, test=0.95. Example predictions for the cellularity radio-pathomic model are presented in Figure 2. Predicted values illustrate the ability for the radio-pathomic model to accurately identify regions of heightened cellularity, even in hypercellular areas beyond the traditional tumor boundary (i.e., contrast enhancement). Example predictions for the same regions are also presented for CYT in Figure 3, and ECF for Figure 4. Despite less variance in these features across tissue samples when compared to cellularity, their respective models are relatively capable in tracking differences in CYT and ECF.Discussion
The presented results show that radio-pathomic modeling using autopsy tissue samples as ground truth can predict histopathological features of brain cancer. In this initial study, accurate predictions were achieved for cellularity, CYT, and ECF. Despite some indications of overfitting, each model was able to predict its target in test set subjects with decent accuracy given our smaller subject-level sample size. Predictive maps generated from these models are able to provide pathological insight beyond traditional imaging signatures, such as identifying hypercellularity beyond the contrast-enhancing region and the FLAIR hyperintensity in some cases, and delineating areas of edema within hyperintense FLAIR signals using ECF maps. These maps have the potential to improve non-invasive tumor monitoring and defining of the active tumor area, which may aid in more precisely directing localized treatments. Future studies based on this work include investigations of diagnostic differences in predictive modeling, as well as incorporating higher order features of the histology (i.e., cell size, shape irregularities). Furthermore, radio-pathomic models could also be used to predict immunohistochemical signatures such as those for mitotic activity (Ki67), as well as localized genetic signatures such as those indicating treatment resistance.Despite the promising results seen in this study, the clinical heterogeneity of this data set motivates further replication in larger data sets in order to provide more robust predictions. Additionally, the time between last MRI scan and death may influence these results, and future studies are necessary to investigate how this time period may influence radio-pathomic relationships.
Conclusion
MP-MRI-based radio-pathomic models are able to predict histopathological features of brain cancer. By developing models from aligned autopsy tissue, these models account for treatment effects, and allow for valid predictions beyond the contrast-enhancing region.Acknowledgements
No acknowledgement found.References
1. Smits M, van den Bent MJ. Imaging Correlates of Adult Glioma Genotypes. Radiology. 2017;284(2):316-331. doi:10.1148/radiol.2017151930
2. Shukla G, Alexander GS, Bakas S, et al. Advanced magnetic resonance imaging in glioblastoma: a review. Chinese Clin Oncol. 2017;6(4):40. doi:10.21037/cco.2017.06.28
3. Husstedt HW, Sickert M, Köstler H, Haubitz B, Becker H. Diagnostic value of the fast-FLAIR sequence in MR imaging of intracranial tumors. Eur Radiol. 2000;10(5):745-752. doi:10.1007/s003300050997
4. Artzi M, Aizenstein O, Jonas-Kimchi T, Myers V, Hallevi H, Ben Bashat D. FLAIR lesion segmentation: application in patients with brain tumors and acute ischemic stroke. Eur J Radiol. 2013;82(9):1512-1518. doi:10.1016/j.ejrad.2013.05.029
5. Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8(35):59492-59499. doi:10.18632/oncotarget.17752
6. Chenevert TL, Malyarenko DI, Galbán CJ, et al. Comparison of Voxel-Wise and Histogram Analyses of Glioma ADC Maps for Prediction of Early Therapeutic Change. Tomogr (Ann Arbor, Mich). 2019;5(1):7-14. doi:10.18383/j.tom.2018.00049
7. LaViolette PS, Mickevicius NJ, Cochran EJ, et al. Precise ex vivo histological validation of heightened cellularity and diffusion-restricted necrosis in regions of dark apparent diffusion coefficient in 7 cases of high-grade glioma. Neuro Oncol. 2014;16(12):1599-1606. doi:10.1093/neuonc/nou142
8. Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol Imaging. 2018;2018:6828396. doi:10.1155/2018/6828396
9. Prah MA, Al-Gizawiy MM, Mueller WM, et al. Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neurooncol. 2018;136(1):13-21. doi:10.1007/s11060-017-2617-3
10. Bobholz SA, Lowman AK, Barrington A, et al. Radiomic Features of Multiparametric MRI Present Stable Associations With Analogous Histological Features in Patients With Brain Cancer. Tomogr (Ann Arbor, Mich). 2020;6(2):160-169. doi:10.18383/j.tom.2019.00029
11. Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538-548. doi:10.1002/jmri.22068
Figures