0661
Clinical evaluation of an AI-accelerated two-minute multi-shot EPI protocol for comprehensive high-quality brain imaging1Siemens Medical Solutions USA, Inc., Boston, MA, United States, 2Department Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Medical Solutions USA, Inc., Atlanta, GA, United States, 5Department Radiology, Stanford University, Stanford, CA, United States, 6Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 7Department Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
This work integrates a novel machine learning-based reconstruction and optimized magnetization transfer preparation modules with a multi-shot echo-planar imaging acquisition to provide comprehensive whole-brain imaging in two minutes. Neuroradiologist evaluation indicated that the proposed method can produce T2, T2*, T1, FLAIR, and DWI images with SNR, tissue contrast, and lesion conspicuity similar to that of a 10-minute turbo spin echo-based exam. To accommodate a wide range of radiologist preferences and/or hardware configurations without the need for additional training, the proposed method provides a tunable parameter for controlling the level of denoising.
Introduction
Achieving rapid, multi-contrast MRI of the brain has been a research goal for over 30 years; however, scan times for high diagnostic quality MR images are still prohibitively long for many clinical scenarios, such as emergency department imaging, where utilization of MRI has been rapidly increasing.1 Traditional turbo spin echo (TSE)-based strategies approach this problem by accelerating acquisitions using parallel imaging,2,3 yet SAR levels and g-factor induced noise amplification ultimately limit these methods to exam times of approximately five minutes. Single-shot echo-planar imaging techniques, with more SAR-efficient encoding, have recently been used to provide comprehensive imaging in 60-90 s, but come at the cost of significant geometric distortion and reduced tissue contrast.4–6In this work we propose an AI-accelerated multi-shot echo-planar imaging (msEPI)-based method that provides high-quality multi-contrast images with high SNR, high tissue contrast, and minimal distortion in two minutes. The method leverages recent advances in machine learning (ML) to improve SNR, magnetization transfer (MT) preparation modules to provide TSE-like contrast, and high per-shot undersampling factors to reduce distortion. A novel training and image reconstruction scheme provides a tunable parameter for controlling the level of denoising/smoothness in reconstructed images, thereby enabling the same neural network to accommodate data with various noise levels. The ability of our neural network-based reconstruction to generalize to pathology, preserving fine details and lesion contrast, is validated through radiological evaluation of clinical patient images not seen during training.
Methods
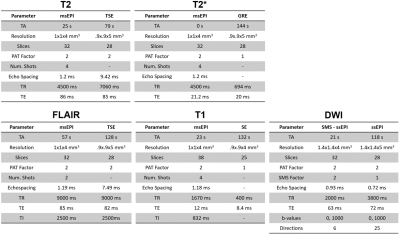
Data AcquisitionThe protocol parameters of the proposed fast msEPI prototype sequences (total acquisition time 2:06 min) and the corresponding clinical reference scans (total acquisition time 10:01 min) are shown in Fig. 1. The prototype sequences (described in [7]) included a combined T2 and T2* acquisition, an inversion recovery-based T1 acquisition, a T2-FLAIR acquisition with a dedicated magnetization preparation module for improved FLAIR contrast, and an SMS diffusion-weighted acquisition.
Data from 5 patients (2 male, 3 female, ages 32-76) and a healthy volunteer were collected on a 3T system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil in accordance with the local IRB and HIPAA. Fully sampled data was collected with 2 averages and 8 shots from another 16 healthy subjects for training (12 subjects) and validation (4 subjects). All patient data was evaluated by two board-certified radiologists.
ML Image Reconstruction
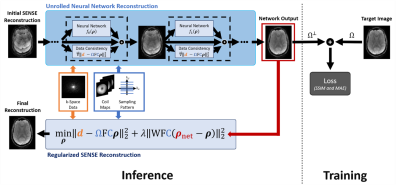
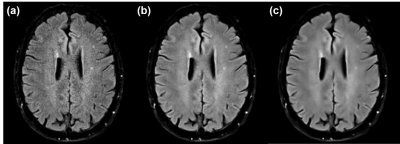
To provide high SNR for the rapid exam, we incorporated machine-learning priors into a regularized SENSE3 reconstruction. Specifically, images were reconstructed by solving the following optimization problem, $${\min_{\mathbf{\rho}}\left\| \mathbf{d} - \Omega\text{FC}\mathbf{\rho} \right\|}_{2}^{2} + {\lambda\left\| \text{WFC}\left( \mathbf{\rho}_{\text{net}} - \mathbf{\rho} \right) \right\|}_{2}^{2},$$ where $$$\mathbf{d}$$$ and $$$\mathbf{\rho}$$$ represent data and image vectors, respectively; $$$\mathbf{\rho}_{\text{net}}$$$ is a neural network-generated image; $$$\text{F}$$$ and $$$\text{C}$$$ are the Fourier transform and coil sensitivity operators; $$$\Omega$$$ is a k-space sampling operator; $$$\lambda$$$ is a regularization parameter; $$$\text{W}$$$ is a diagonal weighting matrix. As a generalization of the work by Hyun et. al,8 this ML-SENSE hybrid reconstruction (illustrated in Fig. 2) provides a tunable parameter ($$$\lambda$$$) for controlling the amount of denoising – a feature that allowed the same network to be used under noise conditions not seen during training (Fig. 3).
For this work, $$$\mathbf{\rho}_{\text{net}}$$$ was generated using an unrolled gradient-descent network (UGDN) with two Down-Up network blocks9 implemented in Pytorch based on the ∑-net package.10 A single UGDN-stage proved sufficient given the low undersampling factor of our protocols. Networks for each contrast were trained following the procedure described in [10]; however, the loss function was modified to incorporate the above regularized reconstruction with $$$\lambda=1.0$$$ and with weights corresponding to sampled lines set to zero, i.e., orthogonal $$$\Omega$$$ and $$$\text{W}$$$ (Fig. 2). This modification allowed training to focus on regions of k-space associated with large weights (e.g. unsampled lines) and better utilize the network’s degrees of freedom. After training, radiologists qualitatively selected the best value of $$$\lambda$$$ for each contrast.
Results
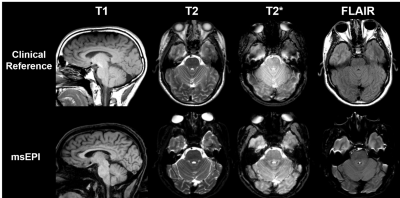
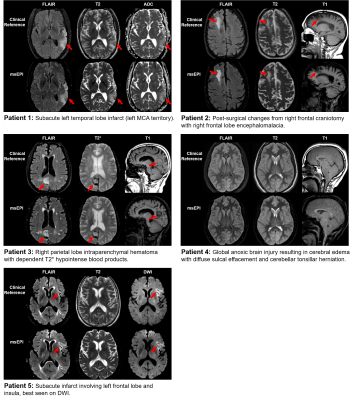
T2, T2*, T1, and FLAIR images were reconstructed using the proposed ML-based method, and GRAPPA2 was used for the DWI to save computation time. Compared to the TSE-based references, msEPI images had significant geometric distortions of the soft facial tissues, but distortion of the temporal lobes and the pons was mild (Fig. 4). Reconstructions of the patient data are shown in Fig. 5. An essential component of our msEPI FLAIR acquisition was an optimized MT preparation module. TSE FLAIR contrast depends strongly on MT,11 and the incorporation of this preparation module provided gray-white contrast and lesion conspicuity similar to the clinical reference.7 Neuroradiologist evaluation revealed that the proposed AI-accelerated msEPI approach preserved fine details and provided similar lesion conspicuity and diagnostic quality compared to clinical reference scans.Conclusions
A novel machine learning-based reconstruction and optimized MT preparation modules have been integrated with a msEPI acquisition to provide comprehensive whole-brain imaging in two minutes, while preserving SNR, tissue contrast, and lesion conspicuity similar to that of a 10-minute TSE-based protocol. By providing a tunable parameter for controlling the level of denoising, the proposed method can accommodate a wide range of radiologist preferences and/or hardware configurations without the need for additional training.Acknowledgements
No acknowledgement found.References
[1] Raja, A. S. et al. Radiology Utilization in the Emergency Department: Trends of the Past 2 Decades. Am. J. Roentgenol. 203, 355–360 (2014).
[2] Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 47, 1202–1210 (2002).
[3] Pruessmann, K. P., Weiger, M., Scheidegger, M. B. & Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 42, 952–962 (1999).
[4] Delgado, A. F. et al. Diagnostic performance of a new multicontrast one‐minute full brain exam (EPIMix) in neuroradiology: A prospective study. J. Magn. Reson. Imaging 50, 1824–1833 (2019).
[5] Skare, S. et al. A 1-minute full brain MR exam using a multicontrast EPI sequence: A 1-Minute Full Brain MR Exam. Magn. Reson. Med. 79, 3045–3054 (2018).
[6] Ryu, K. H. et al. Clinical feasibility of 1-min ultrafast brain MRI compared with routine brain MRI using synthetic MRI: a single center pilot study. J. Neurol. 266, 431–439 (2019).
[7] Conklin, J. et al. A comprehensive multi-shot EPI protocol for high-quality clinical brain imaging in 3 minutes. Proc. Intl. Soc. Magn. Reson. Med. 6691 (2020).
[8] Hyun, C. M., Kim, H. P., Lee, S. M., Lee, S. & Seo, J. K. Deep learning for undersampled MRI reconstruction. Phys. Med. Biol. 63, 135007 (2018).
[9] Yu, S., Park, B. & Jeong, J. Deep Iterative Down-Up CNN for Image Denoising. Proc. IEEE Conf. Comput. Vis. Pattern Recognit. 9 (2019).
[10] Hammernik, K. et al. Σ-net: Systematic Evaluation of Iterative Deep Neural Networks for Fast Parallel MR Image Reconstruction. ArXiv191209278 Cs Eess (2019).
[11] Melki, P. S. & Mulkern, R. V. Magnetization transfer effects in multislice RARE sequences. Magn. Reson. Med. 24, 189–195 (1992).
Figures