0658
Visualization technique for assessment of spinal cord fMRI data quality
Kimberly J Hemmerling1,2 and Molly G Bright1,2
1Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL, United States, 2Physical Therapy & Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
1Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL, United States, 2Physical Therapy & Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Synopsis
It is important to perform visual inspection of spinal cord imaging data throughout an fMRI analysis pipeline. The method presented here is an extension of an existing technique developed for the brain, adapted to spinal cord fMRI. We create a two-dimensional heatmap of the spinal cord derived from four-dimensional imaging data, which can be co-visualized with traces of motion and physiological signals, and identify examples of structured variations in the heatmap that may be attributed to these nuisance signals. Implementing this visualization of spinal cord fMRI data is a simple and fast method to examine data quality.
Introduction
fMRI analysis benefits from visual inspection of images to gain insight into data quality. In fMRI of the brain, there is a method to segment the brain into regions and organize those data onto a two-dimensional ‘gray plot’1. This method has been used extensively in the fMRI community to consider head movement biasing blood-oxygenation level dependent (BOLD) signal changes2, to assess head motion during a scan for quality control34, or to evaluate denoising approaches5. This technique was developed for brain data, using established software packages (such as Freesurfer) that depend on segmentation of brain tissue, and cannot be directly applied to spinal cord (SC) fMRI due to differences in anatomy and data analysis priorities. We extend this technique to provide a method for qualitative visual analysis of SC fMRI. The SC is segmented into appropriate regions, and the voxel timeseries of those regions are presented as a two-dimensional signal intensity heatmap. Recorded physiological signals, estimated rotational and translational motion artifacts, and regression statistics can be co-visualized. Here we present the method, explore options for anatomical organization of the plot, and identify structured variations that may be attributed to features of the co-visualized traces.Methods
The SC is first organized into anatomical regions of interest (e.g., tissue class or vertebral level) using a SC fMRI image registered to the PAM50 template space6 and template masks of SC tissues and vertebral levels warped to the original functional space. The bash script written to do this combines functions from the Spinal Cord Toolbox7, FSL8, and AFNI9. SC masks are first binarized and then iteratively eroded to create concentric masks of the cerebral spinal fluid (CSF), white matter, and gray matter. The timeseries from each voxel in the masks are subsequently extracted and stored.A MATLAB (MathWorks, Natick, MA) script is used to perform numerous data visualizations. Voxel timeseries are arranged as a two-dimensional heatmap with TRs across the horizontal axis, and the selected cord organization on the vertical axis – either by tissue type or longitudinally, by vertebral level and slice (Figure 1). Along with the heatmap, physiological traces such as end-tidal CO2 (PETCO2) or heart rate (HR), and traces of translational and rotational motion can be plotted. T-statistics calculated from a generalized linear model (GLM) can be co-visualized on a voxel-wise basis alongside the heatmap. The heatmap can be shuffled by t-statistic magnitude to highlight different confounds. Additionally, these data can be viewed in the frequency domain, rather than the time domain, to better visualize periodic signal effects typical of certain noise confounds or task paradigms.
To validate this technique, 10 preprocessed SC fMRI datasets are analyzed as described above. Preprocessing includes physiological noise correction (RETROICOR), motion correction, and registration to the template space. Functional datasets with resting state and breath-hold (BH) protocols are compared using this method. We identify and present examples of structured variation in these scans.
Preliminary code and example data are available at https://github.com/BrightLab-ANVIL/spinalcordplot.
Results
Heatmaps organized by tissue type and by vertebral level are presented in Figure 2 with corresponding rotational and translational motion timeseries. The motion artifacts seen here are clearly affected by the organizational method. Figure 3 shows the full output of our technique, including the heatmap, physiological and motion traces, alongside t-statistics output from a GLM. Figure 4 shows a BH scan heatmap visualized in three ways: organized by tissue type, arranged by the magnitude of the HR t-statistics, and arranged by the magnitude of the PETCO2 t-statistics. The structured variance induced by the task is visible in all three, though appearance varies. Heatmaps in the frequency domain of a BH scan, organized both by tissue type and by vertebral level, show that much of the change in BOLD signal across the SC aligns with the frequency content of the physiological traces (Figure 5).Discussion and Conclusion
Implementing these visualizations into an analysis pipeline is a simple way to gain insight into SC fMRI data quality. Inherent SC anatomy established options for arrangement of the heatmap that may affect the final interpretation of noise components. Figures 2 and 4 demonstrate the importance of considering the organizational method when conducting these analyses. Cord motion can have an influence on signal based on the longitudinal axis location or by the tissue region (Figure 2). These findings are in contrast to the organization used for heatmaps of the brain, for which the data are sorted concentrically from the cortical ribbon to the CSF1. Although there is an option to include CSF data in our heatmaps, timeseries of these voxels can dominate the heatmap variance and impact our visualization within the cord itself.The alignment of variation in signal intensity in the heatmaps with variation in the PETCO2 and HR traces is expected due to the task driven nature of the BH scan (Figures 4 and 5). These visualizations highlight the importance of considering the possibility of collinearity between regressors when using a GLM for analysis. Regardless of the method chosen for the organization of the heatmap, this tool provides valuable qualitative insight into SC fMRI data and can be readily incorporated at any stage of an SC fMRI analysis pipeline.
Acknowledgements
Research supported by the Craig H. Neilsen Foundation (595499).References
- Power JD. A simple but useful way to assess fMRI scan qualities. Neuroimage. 2017;154(June 2016):150-158. doi:10.1016/j.neuroimage.2016.08.009
- Gratton C, Dworetsky A, Coalson RS, et al. Removal of high frequency contamination from motion estimates in single-band fMRI saves data without biasing functional connectivity. Neuroimage. 2020;217:116866. doi:10.1016/j.neuroimage.2020.116866
- Fair DA, Miranda-Dominguez O, Snyder AZ, et al. Correction of respiratory artifacts in MRI head motion estimates. Neuroimage. 2020;208:116400. doi:10.1016/j.neuroimage.2019.116400
- Li J, Kong R, Liégeois R, et al. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage. 2019;196:126-141. doi:10.1016/j.neuroimage.2019.04.016
- Glasser MF, Coalson TS, Bijsterbosch JD, et al. Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. Neuroimage. 2018;181:692-717. doi:10.1016/j.neuroimage.2018.04.076
- De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165:170-179. doi:10.1016/j.neuroimage.2017.10.041
- De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24-43. doi:10.1016/j.neuroimage.2016.10.009
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. doi:10.1016/j.neuroimage.2011.09.015
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10(4-5):171-178. doi:10.1002/(SICI)1099-1492(199706/08)10:4/5<171::AID-NBM453>3.0.CO;2-L
Figures
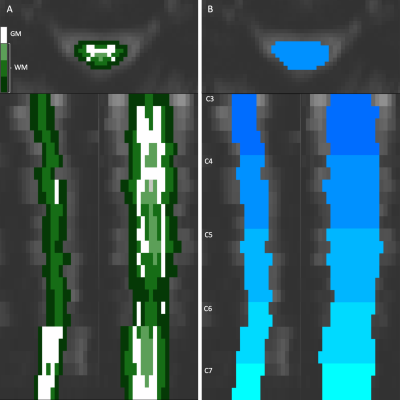
Examples of the two approaches to anatomical organization of the vertical axis of the heatmaps shown in the axial, coronal, and sagittal planes. (A) The cord can be segmented concentrically by tissue type (WM: white matter, GM: gray matter). (B) The cord can be organized by the vertebral level and slice. This example shows the cervical spine between C3 and C7. Color schemes like these will be used alongside the following heatmaps to denote approximate anatomical locations.
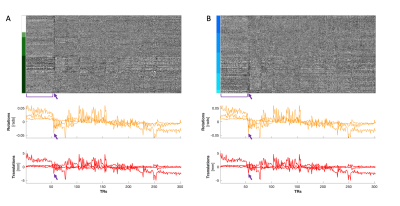
(A) Heatmap organized by tissue type and (B) by vertebral level (C3-C7) in a BH scan. Two structures of motion are notable in these plots: a more prolonged movement indicated by the bracket that appears predominantly in the Rz and Ty motion traces, and a brief movement indicated by the arrows that is most pronounced in Rx, Rz, and Ty. The prolonged movement is discernible in GM and in WM tissues. The brief movement appears to have a more continuous gradation along the y-axis when organized by vertebral level, and the signal increases in magnitude from C3 to C7.
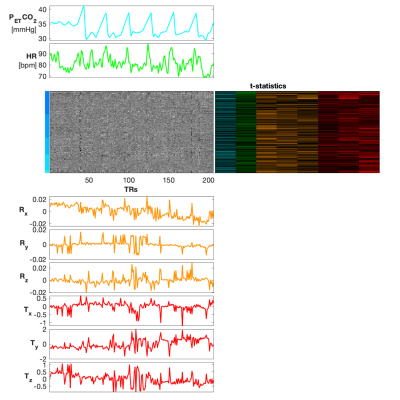
Heatmap organized by vertebral level (C3-C7), co-visualized with physiological traces of PETCO2 and HR, rotation (radians) and translation (mm) motion traces, and voxelwise t-statistics derived from a GLM (PETCO2 and HR were demeaned and convolved with the hemodynamic and cardiac response functions, respectively for the GLM). T-statistic columns are shown for all physiological and motion traces in this figure (PETCO2, HR, Rx, Ry, Rz, Tx, Ty, Tz), and appear in the same order. For simplicity of the color scale, the absolute values of the t-statistics are shown, ranging from 0 to 5.
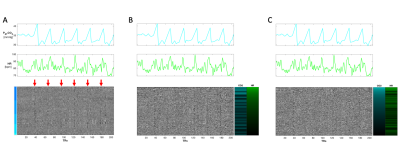
Three heatmaps of a BH scan organized first by (A) vertebral level (C3-C7). Red arrows indicate the location of BHs which coincide with the PETCO2 and HR traces above the heatmap. (B) Heatmap reorganized by GLM derived t-statistics for HR. (C) Heatmap reorganized by GLM derived t-statistics for PETCO2. Motion regressors were also included in the GLM but are not shown here. For simplicity of the color scale, the absolute values of the t-statistics are shown. Though the organization is changed, the structured variation from the BH paradigm is visible in all three heatmap variations.
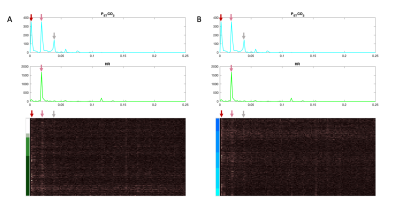
Frequency heatmaps organized by (A) tissue type and by (B) vertebral level. A different color map is used here to indicate the frequency domain, rather than the time domain. Physiological frequency traces are co-visualized above the heatmaps. The frequency axis is bounded by the Nyquist frequency of the scan (0.25 Hz). Frequency content of the fMRI data and the physiological traces clearly have a few similar prominent peaks in common, as indicated by the arrows, and appears to have a more continuous gradation when organized by tissue region.