0635
Mapping digit-representations in BA3b during stimulation and investigating their intrinsic connectivity at rest using VASO1Cognitive Neuroscience, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
While inter-digit interactions are crucial for manual abilities like tool use or object manipulation, individual digits seem to be distinctly represented in the primary somatosensory cortex. Here, we used cortical depth-resolved high-resolution CBV-measurements to investigate these representations in the putative S1-subregion “BA3b” and depth-dependent temporal correlation during rest, as a potential index for layer-specific integration between digit representations. We found that we can
- identify individual digit-representations in human subjects during stimulation using VASO
- show more specific depth profiles for VASO than BOLD
- see intrinsic interactions between digits at rest, which might be driven by deep-to-deep layer connections
Introduction
Inter-digit interactions are crucial for manual abilities like tool use or object manipulation5. However, digits seem to be distinctly represented in subareas of the primary somatosensory cortex (S1)2. In non-human primates, interactions between digits have been demonstrated within and between representations in subregions of S1 during stimulation and rest8. However, there is little evidence on the fine-grained mechanisms behind these interactions and whether similar processes can be found in humans. One outstanding issue with respect to the application of this research to humans is the necessary specificity of non-invasive (fMRI) measurements. In contrast to the commonly used BOLD-signal, which is heavily biased towards the pial surface, slice-selective slab-inversion vascular space occupancy (VASO3) might offer an alternative due to its increased specificity. Therefore, we aim to:- Use VASO to map individual digit-representations in human subjects during stimulation
- explore intrinsic interactions between digit representations at rest.
Methods
Data-acquisition: 4 subjects underwent scanning using a "classical" 7T Magnetom whole body scanner (Siemens Healthineers, Erlangen, Germany), equipped with a Rx 32-channel head coil at Scannexus (Maastricht, The Netherlands). All functional scans were performed with 3D-EPI7, VASO3 with a nominal resolution of 0.75x.075x1.29mm (22 slices TI1/TI2/TR/TE=50/650/2269/25 ms, partial fourier factor=6/8, flip angle=4°, bandwidth=1064Hz/Px, FoV=122mm). Slice position and orientation were chosen individually for each subject and covered the entire postcentral gyrus while optimising resolution perpendicular to its anterior bank (similar to the approach of Huber at al.4 in M1). Finally, 3 high-resolution anatomical images (0.5mm isotropic, 60 slices, TI1/TI2/TR/TE=900/2750/6000/4.02 ms, flip angle1=6°, flip angle2=7°, bandwidth=140Hz/Px, acceleration factor = 2, FoV=160mm) were acquired with the same coverage, using MP2RAGE6.Paradigm: During stimulation runs (~12 minutes), 3 digit-tips (left hand index to middle) were stimulated (30s on-off block design, 4 repetitions/digit) by means of a piezoelectric vibrotactile stimulator (mini PTS system, Dancer Design, UK). During subsequent resting-state scans, subjects were instructed to lay comfortably with their eyes open and to not fall asleep.
Data-processing: Motion- and BOLD-correction was applied using ANTs-Registration and LayNii, respectively. A GLM-analysis was performed in FSL, regions ROIs were manually drawn in FSLeyes and layering and signal extraction was performed on spatially upsampled data (0.1x0.1x1.29mm). Finally, resting-state data was analyzed using custom python-scripts (Nilearn). For analysis code, see: https://github.com/sdres/s1_anfunco
Results and discussion
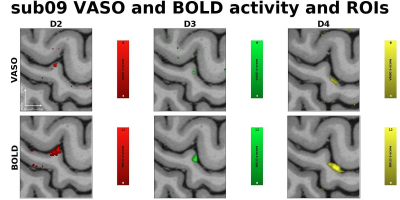
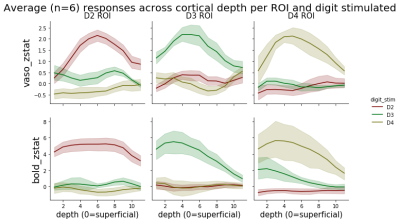
Fig. 1 shows zmaps of a representative subject and resulting ROIs. The VASO activity is spatially specific to the grey matter, while BOLD is more biased towards cerebrospinal fluid (CSF). We were able to define single-slice ROIs based on the VASO compartment of the data for 3 out of 4 subjects.Fig. 2 shows the layer-profiles per ROI and modality across all subjects. Regarding the ROIs corresponding to the stimulated digit, profiles show a peak in middle layers for VASO- and a peak drawn towards the lower cortical depth for BOLD-signal. In VASO, this likely reflects increased input to the granular layer from the thalamus, while the BOLD-signal is dominated by draining veins on the pial surface. The higher sensitivity of BOLD can be seen in markedly higher z-values.
The left matrix in Fig 3 shows correlations between layers within and across ROIs during rest. Highest correlations can be observed between deep to deep cortical depths in all digit-combinations and for superficial to superficial cortical depths between D2 and D3. To investigate the reliability of these initial results, we divided the average matrix by the standard deviation across subjects (right matrix in Fig. 3). Crucially, the regions of higher correlations overlap with the regions of high stability. These initial results hint towards lateral connections between deep layers of digit-representations within BA 3b.
Conclusion and summary
Here, we show the applicability of SS-SI VASO in the investigation of digit-specific representations in S1. Furthermore, we demonstrated the specificity of the technique by showing that, in contrast to the BOLD-signal, activation-peaks can be seen in middle layers, as expected based on input-output characteristics of the canonical microcircuit1. Finally, we provide initial results towards the implication of deep-to-deep layer connectivity between neighbouring, but functionally related patches of cortex.Acknowledgements
- We thank Sriranga Kashyap for his help regarding Image registration.
- Laurentius Huber was funded form the NWO VENI project 016.Veni.198.032
- Ethics: The scanning procedures have been approved by the Ethics Review Committee for Psychology and Neuroscience (ERCPN) at Maastricht University, following the principles expressed in the Declaration of Helsinki.
References
- Felleman DJ, Van Essen DC Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991; 1(1): 1–47.
- Jones, EG, Coulter JD, & Hendry SHC Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. Journal of Comparative Neurology. 1978; 181(2): 291-347.
- Huber L, Ivanov D, Krieger SN, et al. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio. Magn. Reson. Med. 2014; 72: 137–148.
- Huber L, Finn ES, Handwerker DA, et al. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. NeuroImage. 2020; 208
- Hsiao S. Central mechanisms of tactile shape perception. Current Opinion in Neurobiology. 2008; 18(4): 418–424.
- Marques JP, Kober T, Krueger G, et. al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010 49: 1271–1281.
- Poser BA, Koopmans PJ, Witzel T, et al. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage. 2010; 51(1): 261–266.
- Wang Z, Chen LM, Négyessy L, et al. The Relationship of Anatomical and Functional Connectivity to Resting-State Connectivity in Primate Somatosensory Cortex. Neuron 2013; 78(6): 1116–1126.
Figures
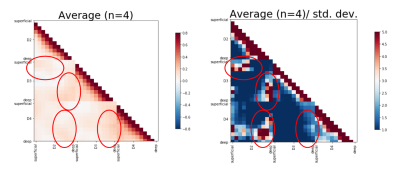
Layer to layer correlations across ROIs. Left: Average connectivity matrix across 4 subjects. Increased correlations are highlighted by red ellipses. Specifically, deep layers of a given digit representation seem to be intrinsically more connected to deep than other layers of other digits' representations. Furthermore, superficial layers of D2 and D3 show a similar pattern.
Right: In order to investigate the stability of these findings, we divided the average matrix by the std. deviation across subjects. Crucially, regions of higher correlations also show highest stability.