0632
VASO-fMRI with Nordic-PCA for laminar sensory testing at 7 Tesla1Institute of Neuroscience & Psychology, University of Glasgow, Glasgow, United Kingdom, 2NIH, Bethesda, MD, United States, 3Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
The most commonly used contrasts in laminar fMRI are blood-oxygen-level-dependent (BOLD) and vascular-space-occupancy (VASO). However, at laminar resolution, brain activity is often buried under noise, complicating the detection of small changes in activation.Here, we show the successful extraction of laminar brain activity of a complex-valued BOLD- and VASO-fMRI time series during a somatosensory task using NORDIC-PCA denoising. We expect this method to be a great asset for laminar sensory fMRI experiments as it reduces the need for anatomically informed smoothing or anisotropic filtering, which might be helpful for very small voxel sizes or when small activated areas are studied.
Introduction
Blood-oxygen-level-dependent (BOLD) and vascular-space-occupancy (VASO) based functional magnetic resonance imaging (fMRI) are the most commonly used contrasts in laminar fMRI. However, at laminar resolution, brain activity is often buried under noise, complicating the detection of small changes in activation. The Noise Reduction with Distribution Corrected (NORDIC) principal component analysis (PCA) method has been shown to increase temporal Signal-To-Noise Ratios (tSNR) when processing fMRI time series (Vizioli et al., 2020). Here, we show that the signal gains from NORDIC can be used to detect laminar VASO and BOLD fMRI activation in the somatosensory cortex in response to weak sensory stimuli such as pin-pricks.Methods
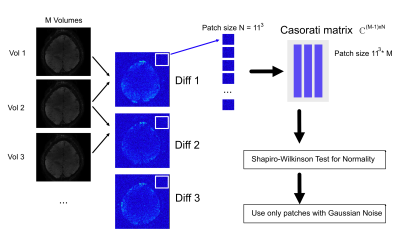
All fMRI data were acquired on a 7T Magnetom TERRA system (Siemens Healthineers, Erlangen, Germany) with a 32-channel Head Coil (Nova Medical Inc, MA, USA). Two subjects were scanned after obtaining research board approval and written consent according to local rules (project no. 302122). A segmented 3D echo-planar imaging (EPI) VASO sequence was used (Stirnberg et al., 2017) (Subject 1: TR/TE 7340/17 msec, matrix 240x240, resolution 0.62x0.62x0.9 mm3, variable flip angles (first angle 60 degrees), 2 segments per kz-shot; Subject 2: TR/TE 3511/19 msec, matrix 188x188, resolution 0.78x0.78x0.9 mm3, variable flip angles (first angle 60 degrees), 1 segment per kz-shot). An MRI-safe and 7T compatible calibrated pin-prick set was used as sensory stimulus, applied by one of the experimenters (MRC Systems, Heidelberg, Germany). A block design was used with 35 seconds rest, followed by 35 seconds task with 10 repetitions. For subject 2, a finger tapping task was used with same timing. Data were reconstructed offline using the Gadgetron framework (Hansen and Sorensen, 2013). A region of interest (ROI) in the motor cortex was used to extract activity patterns for BOLD and VASO. The original NORDIC-PCA method requires a scan without radio frequency (RF) excitation to estimate the noise variation, which was not acquired in this experiment. To apply NORDIC without this extra scan, the variation was estimated from the complex difference of successive fMRI volumes as described in Figure 1. Volumes were divided into Casorati patches and tested for Gaussian normality using a Shapiro-Wilkinson test. From the patches with Gaussian noise, the NORDIC variance of the original time series was then estimated as Var(X-Y) = Var(X) + Var(Y).Results
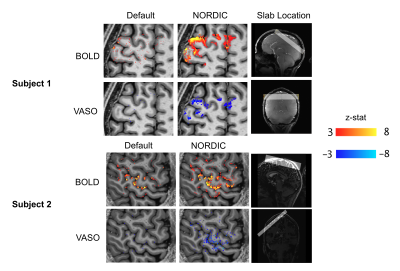
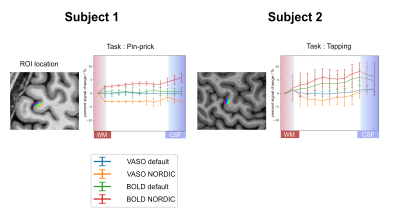
Compared to the default reconstruction, NORDIC successfully reduced the noise for both BOLD and VASO, especially the signal seen in the motor and premotor cortex (Figure 2). Using NORDIC, laminar activity induced by the pin-prick stimulus was robust, showing a large improvement for both BOLD and VASO activity. Signals for this task peaked in the upper layers for BOLD and in the middle layers for VASO. The finger tapping task showed a clear contrast for both BOLD and VASO using a default reconstruction. However, statistical significance dramatically increased using NORDIC, particularly for VASO maps.Figure 3 shows the calculation of laminar profiles for BOLD and VASO. In the case of the pin-prick stimuli, BOLD and VASO activation remained in the noise floor and the laminar profiles were not significantly different from zero. BOLD activation was maximal in the upper layers while VASO was maximal in the middle of the cortex. For the finger-tapping stimulus, both with and without NORDIC, a laminar functional profile was obtained with BOLD peaking in upper layers and VASO peaking in middle layers.
Discussion
We show that denoising the VASO signal using NORDIC increases the SNR and allows extraction of more robust laminar functional profiles in the human brain. Detecting weak brain activity at laminar resolution in healthy subjects has proven to be difficult and is expected to be even more complicated in patients. This is where we expect this method to be a great asset. It could reduce the need for anatomically informed smoothing or anisotropic filtering which might be especially helpful for very small voxel sizes or when laminar profiles in small activated areas are studied.Acknowledgements
We are grateful to Rüdiger Stirnberg for his support with the 3D-EPI sequence, which forms the basis for the VASO readout. We also thank Yulia Lazarova and Jasper Fabius and for their support during the MRI acquisitions.
Funding: Nils Nothnagel, John Riddell and Jozien Goense are funded by the Medical Research Council (MR/R005745/1). Andrew T. Morgan is funded by the Medical Research Council (MR/N008537/1) and the European Union’s Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 and 945539 (Human Brain Project SGA2 and SGA3). Laurentius Huber was funded form the NWO VENI project 016.Veni.198.032.
References
- Hansen, M. S. & Sorensen, T. S. 2013. Gadgetron: an open source framework for medical image reconstruction. Magn Reson Med, 69, 1768-76.
- Stirnberg, R., Huijbers, W., Brenner, D., Poser, B. A., Breteler, M. & Stocker, T. 2017. Rapid whole-brain resting-state fMRI at 3 T: Efficiency-optimized three-dimensional EPI versus repetition time-matched simultaneous-multi-slice EPI. Neuroimage, 163, 81-92.
- Vizioli, L 2020. A Paradigm Change in Functional Brain Mapping: Suppressing the Thermal Noise in fMRI. bioRxiv doi: https://doi.org/10.1101/2020.11.04.368357
Figures