0630
Whole brain layer-fMRI: An open dataset for methods benchmarking1Goethe-Universität Frankfurt am Main, Mainz, Germany, 2Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Scannexus, Maastricht, Netherlands, 4Brain Innovation, Maastricht, Netherlands, 5NIH, Bethesda, MD, United States
Synopsis
Laminar-specific fMRI allows neuroscientists to address research questions of directional functional connectivity within and across brain areas. While recent sequence developments allow considerable improvements in coverage, resolution, and mitigation of venous biases, it is not established how routinely useful those methods are for everyday neuroscientific application.
We present an open dataset of whole-brain CBV-sensitive layer-dependent fMRI during free movie watching. Its purpose is to:
- investigate the reproducibility of laminar connectivity results,
- to benchmark preprocessing pipelines,
- to provide reference atlases of laminar connectivity and venous physiology,
- and to explore new potential neuroscientific research questions that become addressable with laminar fMRI.
Purpose
Laminar-specific fMRI allows neuroscientists to address research questions of directional functional connectivity within and across brain areas. While recent sequence developments allow considerable improvements in coverage, resolution, and mitigation of venous biases, it is not established how usable those methods are for routine neuroscience praxis.Here, we provide a whole-brain layer-dependent functional connectome dataset with cerebral blood volume and BOLD contrast. The purpose of this dataset is to:
- characterize the challenges of whole brain layer-fMRI acquisition sequences in a test-retest setting and to see which neuroscientific research questions are reliably addressable and which ones aren’t.
- provide a test bed for developing and benchmarking new layer-dependent preprocessing and analysis tools.
- provide an atlas of laminar connectivity and depth-specific vascular physiology (vascular reactivity).
- characterize the layer-dependent representation content across all areas.
Methods
Scanning was performed on a SIEMENS MAGNETOM “Classic” 7T scanner while subjects were watching the Human Connectome Project (HCP) audio movie. For layer-fMRI scanning without venous biases, a blood volume sensitive VASO (Lu 2003) sequence was used. We further developed a previously presented MAGEC VASO variant with an inversion T1-primer to increase the coverage by another 20% compared to previous protocols (Huber 2020).Scanning: Resolution = 0.8mm iso, TR = 8.9s, 3D-EPI readout (Poser 2010), in-plane GRAPPA 3, TA = 14 min, 4-5 runs per session and 6 sessions in total (one of which failed). For further acquisition parameters see: https://github.com/layerfMRI/Sequence_Github/blob/master/Whole_brain_layers/Ann_WB_VASO_fMRI.pdf. Respiration and pulse traces were recorded to provide the required physiological measures for further analysis.
Data processing: Motion correction and VASO's BOLD correction was performed in LayNii. Segmentation of GM and WM were initially performed in BrainVoyager and then manually corrected. To estimate the functional sensitivity to extract brain networks, ICA was used and the number of ‘neural’ vs. ‘non-neural’ were counted for various layer-specific smoothing strengths.
Venous baseline CBV was extracted by means of the recorded respiratory volume using the VasA approach (Kazan 2017; Guidi 2020).
The data are openly accessible on OpenNeurio: https://10.18112/openneuro.ds003216.v1.0.0. We are happy to share the used sequence via SIEMENS C2P.
Results and Discussion
Figs. 1 and 2 show that common acquisition and analysis procedures that work for conventional resolutions and small FOV laminar fMRI do not suffice for whole brain laminar fMRI. With the long EPI echo spacing (low bandwidth), which is necessary with the large matrix sizes, the common rigid body motion-correction across runs and sessions does not provide sufficient local accuracy. Higher-order non-linear motion correction with ANTs SyN (Avants 2008) becomes necessary. Furthermore, the small bandwidth also makes the data more sensitive to frequency drifts that cause run-consistent traveling-wave artifacts into the time series (Fig. 2), which suggests that laminar-fMRI results need to go though additional temporally shifted detrending (nuisance regression) steps.Fig. 3 shows that the commonly low SNR of laminar fMRI can be accounted for with moderate layer-specific smoothing (Blazejewska 2019), without significantly reducing the laminar specificity.
Fig. 4 depicts a representative atlas of area specific distributions of vascular reactivity (venous baseline blood density) across depth. This atlas will play an important role in future studies using laminar signal models (Markuerkiaga 2016,2020; Havlicek 2019).
Fig. 5 depicts an exploratory analysis method to map brain areas based on their layer-specific information content. The purpose of this figure is to exemplify what kind of new research questions can be addressed with whole brain layer-fMRI datasets like this one that cannot be easily addressed with conventional fMRI protocols. Here we use it for hypothesis testing of two models of brain function: A representational vs. an intentional framework (Shadlen 2013). Conventional two-dimensional brain cartography often operates under the implicit assumption that each brain module's purpose is to ‘represent’ and ‘classify’ features and objects of the outside world. Laminar fMRI allows us to investigate how appropriate this model is. We find indeed that many associative areas seem to be also well characterized based on a ‘non-representational’, but by an ‘intentional’ framework (Shadlen 2013).
Summary and Conclusion
Here we present an open dataset for benchmarking laminar fMRI acquisition, for testing and optimizing preprocessing strategies, and for characterizing the reproducibility of layer-fMRI neuroscience results. We find that upon appropriate preprocessing, common connectivity networks can be reliably obtained with local specificity to the laminar profiles. The exceptionally high number of identical runs in this dataset allow us to provide representational atlases of venous baseline blood volume distribution.These data were further used to estimate a ‘column-by-column’ level characterization of whether or not a brain area is dominated from externally-driven feedforward activity or intentionally-driven endogenious activity.
Acknowledgements
Help with scanning: These data were acquired with the kind support of scannexus at their 7T MAGENTOM “Classic” SIEMENS scanner. We thank Sebastian Dresbach and Kabir Arora for assistance with after hour scans. We thank Bianka Linssen for advice regarding head stabilization cushions and cardio-respiratory tracing.
Funding:
Anna Katharina Mueller was supported for this project by a fellowship from the Goethe-University in Frankfurt am Main.
Laurentius Huber was funded form the NWO VENI project 016.Veni.198.032.
Benedikt Poser is partially funded by the NWO VIDI grant 16.Vidi.178.052 and by the National Institute for Health grant (R01MH/111444) (PI David Feinberg).
Miriam Heynckes is funded by The Netherlands Organization for Scientific Research (NWO) Research Talent Grant 406.17.200.
Ethics: The scanning procedures have been approved by the Ethics Review Committee for Psychology and Neuroscience (ERCPN) at Maastricht University, following the principles expressed in the Declaration of Helsinki.
References
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. doi:10.1016/j.media.2007.06.004
- Blazejewska AI, Fischl B, Wald LL, Polimeni JR. Intracortical smoothing of small-voxel fMRI data can provide increased detection power without spatial resolution losses compared to conventional large-voxel fMRI data. Neuroimage. 2019;189:601-614. doi:10.1016/j.neuroimage.2019.01.054
- Guidi M, Huber L, Lampe L, Merola A, Ihle K, Möller HE. Cortical laminar resting-state fluctuations scale with the hypercapnic bold response. HBM. 2020;41:2014-2027. doi:10.1002/hbm.24926
- Havlicek M, Uludag K. A dynamical model of the laminar BOLD response. Neuroimage. 2019;204:116209. doi:10.1101/609099
- Huber L, Finn ES, Chai Y, et al. Layer-dependent functional connectivity methods. Prog Neurobiol. 2020:in print. doi:j.pneurobio.2020.101835
- Kazan SM, Huber L, Flandin G, Ivanov D, Bandettini P, Weiskopf N. Physiological basis of vascular autocalibration (VasA): Comparison to hypercapnia calibration methods. Magn Reson Med. 2017;78(3):1168-1173. doi:10.1002/mrm.26494
- Lu H, Golay X, Pekar JJ, van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263-274. doi:10.1002/mrm.10519
- Markuerkiaga I, Marques JP, Gallagher TE, Norris DG. Estimation of Laminar BOLD Activation Profiles using Deconvolution with a Physiological Point Spread Function. bioRxiv. 2020:1-28. doi:10.1101/2020.08.04.236190
- Markuerkiaga I, Barth M, Norris DG. A cortical vascular model for examining the specificity of the laminar BOLD signal. Neuroimage. 2016;132:491-498. doi:10.1016/j.neuroimage.2016.02.073
- Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 tesla. Neuroimage. 2010;51(1):261-266. doi:10.1016/j.neuroimage.2010.01.108
- Shadlen MN, Kiani R. Decision making as a window on cognition. Neuron. 2013;80(3):791-806. doi:10.1016/j.neuron.2013.10.047
Figures
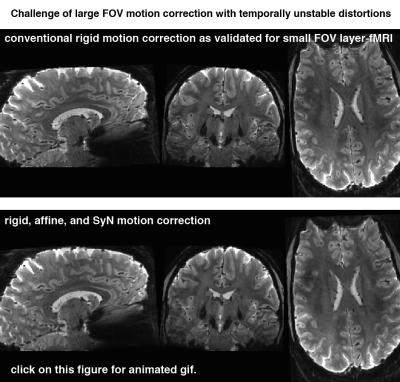
Challenges of non-linear motion:
GIF animation across runs of different days. We find that the conventional preprocessing steps that have been validated and established for small FOV layer-fMRI protocols do not yield acceptable quality. While ANTs’s SyN algorithm can mitigate large parts of the nonlinear motion, it has 289 times larger CPU and 78 times larger memory demands.
Note that variable local signal biases across sessions are stable within sessions and do not limit the functional sensitivity within runs.
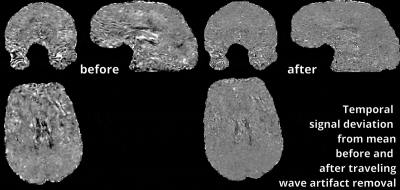
These novel types of data exhibit similarly novel artifacts, which need to be corrected for:
CLICK on figure for animation to start.
The low bandwidth used for large FOV layer-fMRI makes the functional data more prone to faint system instabilities (E.g., slow frequency drifts).
The left panel depicts the signal variations across TRs for the average of 25 runs (within and across days). The right panel depicts the same data after temporal shift regression artifact removal.
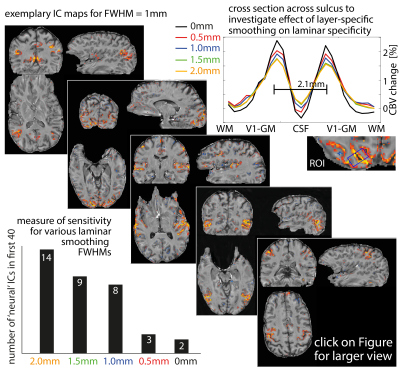
Finding best layer smoothing.
Since layer-fMRI is limited by SNR constraints, it can be hard to extract meaningful networks without spatial smoothing. Here we use ICA and take the nr. of ‘neural’ ICs as a proxy for sensitivity across smoothing strengths (bottom left). The distinguishability of two layer peaks (two GM banks in V1 2.1 mm apart) is used as a proxy for specificity (top right). These quality metrics are compared across laminar specific smoothing strengths.
Diagonals depicts representative ICA-derived connectivity maps.
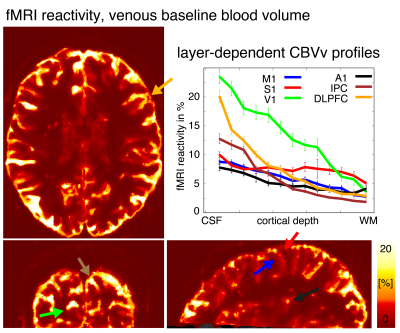
Atlas of baseline CBV profiles.
One important parameter for the interpretation of laminar results and laminar fMRI signal models, is the layer-dependent vascular density (aka. vascular reactivity, venous baseline blood volume). Here we use the temporal variations of the recorded respiratory volume in the low frequency regime as regressors to estimate layer dependent vascular reactivity for all cortical areas. Few exemplary areas with different laminar slopes and magnitude are highlighted.
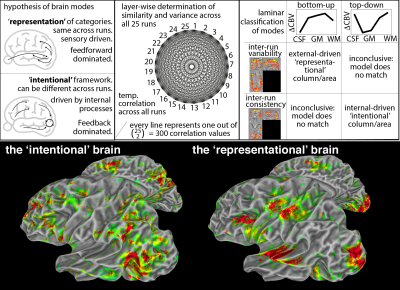
Exploring new research questions addressable with layer-fMRI: The brain as ‘representational’ vs. ‘intentional’ framework:
One influential working hypothesis in neuroscience is that the brain modules represent and classify the outside world (identical for the same stimulus), feedforward driven. However, this does not capture activity modulation based on ‘intentional concepts’ (task instructions, attention, expectation, arousal). Here, we map which of those models better explains layer-fMR profiles.