0623
A 64-Channel Brain Array Coil with an Integrated 16-Channel Field Monitoring System for 3T MRI
Mirsad Mahmutovic1, Alina Scholz1, Nicolas Kutscha1, Markus W. May1, Torsten Schlumm2, Roland Müller2, Kerrin Pine2, Luke J. Edwards2, Nikolaus Weiskopf2,3, David O. Brunner4, Harald E. Möller2, and Boris Keil1
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany, 4Skope Magnetic Resonance Technologies AG, Zurich, Switzerland
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany, 4Skope Magnetic Resonance Technologies AG, Zurich, Switzerland
Synopsis
Combining highly parallel array coils, magnetic field monitoring, and high gradient strength provide a complementary approach to enhance diffusion-weighted imaging (DWI). A 64-channel receive brain array with an incorporated 16-channel field camera system was developed to be used with 300 mT/m gradients from the 3T Connectom scanner. The increased signal-to-noise ratio (SNR) and implemented parallelism was highly beneficial, and will improve SNR-starved high-b value DWI acquisitions, while the magnetic field monitoring data successfully corrected the images from blurring, aliasing and distortions.
Introduction
Diffusion-weighted Imaging (DWI) is a powerful method to reconstruct and visualize brain fiber tracts of the human connectomics. However, it has already been shown that DWI is compromised by field perturbations, mainly caused by eddy currents induced by diffusion-weighting gradients, B0 drift and gradual changes of the short-term gradient system response during the scan1. Related artefacts can be eliminated by incorporating a magnetic field monitoring system that concurrently measures the spatiotemporal magnetic field dynamics during the acquisition of diffusion data1,2. Furthermore, the ability to map the connectivity pathways is limited by the image sensitivity, resolution, and capable acquisition speed. These critical imaging attributes can be greatly improved with dedicated high-density array coils. The aim of this study was the development and the technological orchestration of a new 64-channel brain array coil with an integrated 16-channel field monitoring system combined with high gradient performance of the 3T Connectom dedicated DWI scanner3,4.Methods
Rx array coil: The brain array coil was constructed on an anatomically shaped former (Fig.1a). All coil parts were 3D-printed in polycarbonate plastic (Fortus450mc, Stratasys Ltd., USA). All neighboring elements were decoupled by critical overlap except the two eye loops which were decoupled using a shared conductor design to facilitate large eye cutouts of the housing. The matching network, active and passive detuning circuitry from each element were placed on the preamplifier’s daughter board. Next-nearest neighboring coil elements were decoupled using preamplifier decoupling5 by transforming a high impedance in the loop. Pairs of coils were attached to a twin preamplifier (Siemens Healthcare GmbH, Erlangen, Germany), which were then multiplexed onto one coaxial cable output6. Preamplifier outputs were bundled and passed through cable traps to suppress common mode currents. Bench measurements verified the element tuning, active detuning, neighboring coupling, and preamplifier decoupling for each coil element.Field monitoring: A well-suited distribution of the 16 magnetic field probes (Skope, Zurich, Switzerland) was determined by an iterative process. It started from the optimal probe configuration for the head, defined as an array of three z-stacked rings, each with 4, 6, 5 probes and one probe at the top7,8. Based on constrains employed by the high-density receive coil structure, the field probes were then successively repositioned form the optimal probe configuration to reduce the amount of local B0 field distortions from surrounding RF components. For each desired potential probe distribution, the field camera performance was re-evaluated to maintain a suitable solution set. A shielded interface box of the field camera setup was then integrated into the back-end of the coil housing (Fig.1c).
Data acquisition: The 3T MAGNETOM Connectom (Siemens Healthcare) with 64 receive channels was used for phantom and initial in vivo imaging. Signal-to-noise ratio (SNR), noise correlation and g-factor calculations were obtained from PD-weighted raw data and compared to results from a standard 32ch coil. Initial in vivo data were acquired in a healthy volunteer with both coils including MP-RAGE (1mm isotropic, 3xGRAPPA) as well as series (20 repetitions) of spin-echo (SE) spiral (62 slices, 0.8mm isotropic, 4 interleaves, TE=33ms, TR=6.9s, bandwidth 952Hz/px) and SE-EPI (TE=66ms, TA=8.9s, bandwidth 1148Hz/px) acquisitions. ME-GRE images were recorded (1.5mm isotropic) to calculate B0 and coil sensitivity maps. Spiral data were reconstructed using Skope-i, incorporating the recorded trajectories and B0 and sensitivity maps2,9.
Results
The 64ch coil shows nearest-neighbor decoupling of –17dB and preamplifier decoupling of –20dB. The coupling between non-adjacent coils ranged from –11dB to –27dB. The QU/QL-ratio was 229/49=4.7. The SNR increased by 1.4-fold at cortical locations when compared to the standard 32ch array (Fig 2b) and was roughly identical in the center. Compared to the standard 32ch coil, the 64ch array yielded improved encoding capabilities for highly accelerated imaging for both in-plane acceleration and simultaneous multi-slice (SMS) imaging. The 64ch coil allowed additional in-plane acceleration by 1 unit for a given noise amplification factor (Fig.3). With SMS, the 64ch coil allowed separation of six collapsed slices with minimal noise gain, while the 32ch coil only allowed four slices with identical noise amplification (Fig.4). Results from the in-vivo experiment corroborated the SNR increase achieved with the 64ch coil (Fig.5).Discussion
A number of technical issues arise by combining large channel-count coil arrays with a multichannel magnetic field camera. Both structures share the same real estate on a close-fitting helmet, causing conflicts in the implementation of the optimal configuration for both systems. The 64ch array coil shows substantial gains in SNR and encoding power when compared to a standard 32ch head coil, which will be particularly beneficial for high-b value DWI acquisitions. We experienced faster decay rates from the in-built field probes, which is likely attributed to the local modulation of the susceptibility, caused by surrounding RF components and the plastic housing. We were able to reconstruct spiral images with substantial reduction of blurring, aliasing, and distortions. However, we feel that further investigations will be needed to allow reliable monitoring of higher order field dynamics.Conclusion
A constructed 64-channel head array coil with an integrated 16-channel field monitoring system was designed, constructed, and validated. The coil/camera system enables the combination of dynamic field monitoring with high reception sensitivity and image acceleration capabilities.Acknowledgements
Special thanks to Simon Gross and Christoph Barmet from Skope Magnetic Resonance Technologies AG for the helpful discussions.References
- Wilm B.J, et al. “Diffusion MRI with concurrent magnetic field monitoring.” Magn. Reson. Med. 74 (2015): 925-933.
- Wilm B.J et al. “Single-shot spiral imaging enabled by an expanded encoding model.” Magn. Reson. Med. 77 (2017): 83-91.
- Setsompop K, et al. “Pushing the limits of in vivo diffusion MRI for the Human Connectome Project.” NeuroImage 80 (2013): 220-233.
- McNab, JA, et al. “The Human Connectome Project and beyond: initial applications of 300 mT/m gradients.” NeuroImage 80 (2013): 234-245.
- Roemer, P.B. et al. “The NMR phased array.” Magn. Reson. Med. 16 (1990): 192–225.
- Keil B, et al. “A 64-channel 3T array coil for accelerated brain MRI.” Magnetic Resonance in Medicine 70 (2013): 248-258.
- Barmet C, et al. “Concurrent higher-order field monitoring for routine head MRI: an integrated heteronuclear setup.” Proceedings of the 18th Annual Meeting of ISMRM, Stockholm, Sweden. Vol. 216. (2010).
- Barmet C, et al. “Spatiotemporal magnetic field monitoring for MR.” Magn. Reson. Med. 60 (2008): 187-197.
- Chen, Nan-kuei, et al. "A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE)." NeuroImage 72 (2013): 41-47.
Figures

Constructed 64-channel array coil with an
incorporated 16-channel field camera system. (a) 3D model of the coil, (b)
enclosed finished coil, (c) view into the open coil.
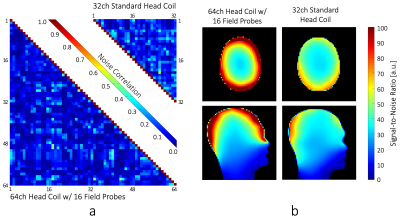
(a) Comparison of the noise correlation: 64ch:
mean: 10.4%, max 51%. 32ch: mean: 15%, max 53%.
(b) SNR comparison of the constructed 64ch coil and the standard 32ch
head coil.
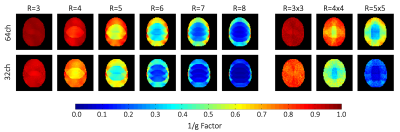
Comparison of the inverse SENSE g-Factors of the
developed 64ch coil and the standard 32ch head coil.
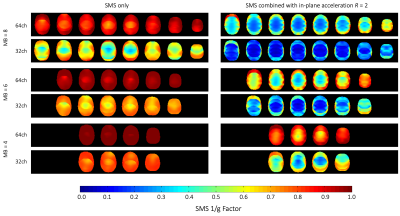
Comparison of inverse SMS g-Factors of the of
the developed 64ch coil and the standard 32ch head coil.
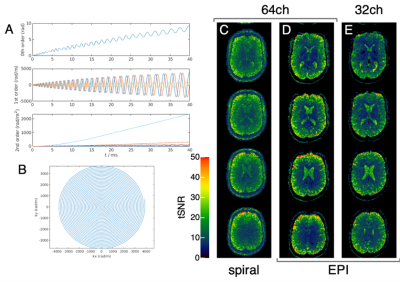
(A) Zero-order phase and higher-order dynamic dynamic field effects; (B)
first-order read trajectory. Examples of tSNR maps (calculated from series of
20 repetitions) obtained with the 64ch coil and (C) spiral as well as (D) EPI
acquisitions. Images reconstructed from the spiral acquisitions using
concurrently monitored field information do not show evident distortions at
visual inspection. (E) Corresponding results obtained with EPI and the standard
32ch coil are shown for comparison. Note the SNR gain obtained with the 64ch
coil, in particular in cortical regions.