0620
Rapid calibration scan for estimating temporally-varying eddy currents in diffusion imaging using a time-resolved PEPTIDE imaging approach1Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
A rapid calibration scan for estimating eddy-currents in diffusion acquisitions was developed using the time-resolved PEPTIDE imaging approach. This calibration scan estimates temporally-varying eddy-current fields across three principal diffusion-directions in <30s, with estimates of eddy-fields across other directions derived through a linear model. The accuracy of this method was validated in simulation, phantom and in-vivo experiments. In a high-SNR diffusion phantom, estimated eddy-fields closely match that of “FSL-eddy” on a large blip-up and blip-down 64-direction EPI dataset. For in-vivo data at b=5000s/mm2, estimates from PEPTIDE-based calibration was able to maintain high-accuracy estimation of the eddy-current field despite low SNR.
Introduction
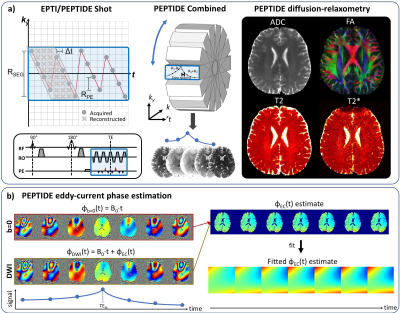
Diffusion imaging is well known to suffer from eddy-currents, manifesting as distortions in EPI1. Many methods for correcting such distortion effects have been proposed2,3,4, with commonly applied techniques relying on estimating the eddy-current fields from a series of distorted images across diffusion-directions5. As an alternative, field-probes6,7 have shown excellent promise for direct eddy-current field measurements, with capability to capture both spatial and temporal variations8,9, but this is specialized hardware and not yet widely accessible.Recently, a distortion- and blurring-free multi-shot EPI technique, termed echo-planar time-resolved imaging (EPTI)10, has been developed, that additionally provides a time-resolved multi-contrast imaging capability. Moreover, EPTI has been combined with a PROPELLER-like readout in the PEPTIDE approach11,12 for self-navigated diffusion-relaxometry imaging (method overview in Figure 1a). These time-resolved imaging approaches avoid the typical image-distortion artifacts from B0 and eddy-current fields, with the phase evolution effects from these fields instead captured in the reconstructed time-series images.
In this work, we exploit this phase evolution information in the PEPTIDE imaging-series to create a rapid eddy-current calibration scan (Figure 1b). Realistic simulations were performed to demonstrate the capability of such an approach to accurately capture both spatial and temporal eddy-current field variations, with an iterative PEPTIDE-reconstruction also developed to ensure robust estimation, even in the presence of strong eddy-currents. This was then further validated through phantom and in-vivo experiments.
Methods
Eddy-field estimation: Estimations can be performed using data from a single PEPTIDE-blade across three principal diffusion directions, with estimates of eddy-fields across other diffusion-directions calculated through a linear model. Estimation for each principal diffusion-direction is performed using the time-series images from that direction and the b=0 reference, which contain the following phase components (Figure 1b):$$\phi_{DWI}(t)=\phi_{background,DWI}+\phi_{B0}(t)+\phi_{EC}(t)$$
$$\phi_{b=0}(t)=\phi_{background,b=0}+\phi_{B0}(t)$$
Across the time-series, the background phase is removed from both datasets and the difference between the two resultant image phases found, to yield the eddy current induced phase. A weighted quadratic fit is then applied per time-point to determine the time-varying phase spatial components. For the case where is assumed to vary linearly with time, a temporal-fit to a constant eddy-field is performed. Assuming a TR of 3.5s, the proposed 3-direction, single-PEPTIDE-blade acquisition requires 3.5s x 4 = 13.5s (including a reference b=0), with an additional PEPTIDE reconstruction reference scan of ~15s13, resulting in a total eddy-current calibration acquisition time of <30s. More blades/directions can be added as needed to improve eddy-field estimation.
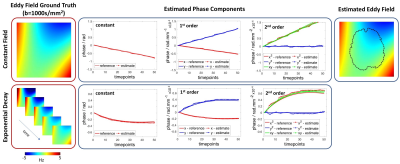
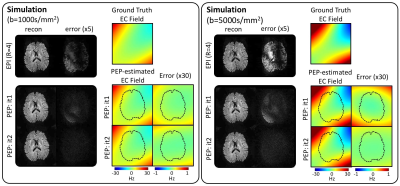
Simulation validation: Simulated PEPTIDE-data for the proposed calibration scan were generated using previously-acquired fully-sampled in-vivo k-t dataset, as previously described11. Here, realistic eddy-current phase changes were also added to the data, using eddy-current fields estimated with FSL eddy5 from EPI-acquisitions with matched parameters. Two cases were simulated: static eddy-field and eddy-field with an exponential temporal decay.
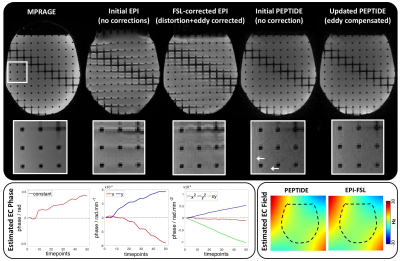
Phantom & in-vivo validation: Phantom and in-vivo datasets were acquired on a Siemens Prisma 3T with a 32-channel coil. A high-SNR diffusion phantom was used for optimal eddy-current estimation and in-vivo data were acquired in a healthy subject. Diffusion-PEPTIDE and diffusion-EPI were both acquired with ESP/TE/TR=1.1ms/128ms/3.5s, 1.5x1.5x3.0mm3, 18-slices, 64 diffusion-directions, b=5000s/mm2. The EPI acquisitions were repeated with reverse phase-encode directions to enable accurate eddy-field estimation with FSL eddy5,14 for use as comparison. PEPTIDE was acquired with 10-blades (with a single blade used for field-estimation) and other parameters as previously reported12.
Results
With the simulated PEPTIDE-data, eddy-current phases were accurately estimated for both static and temporally-varying eddy-current fields cases (Figure 2). The use of a second iteration of the PEPTIDE reconstruction was also investigated, where the estimated eddy-current is incorporated into the B0-GRAPPA PEPTIDE-reconstruction, which is shown to reduce the already low reconstruction errors and further improve the eddy-field estimates (Figure 3). The relatively low errors seen at both b=1000s/mm2 and b=5000s/mm2, supported the resilience of the technique.Figure 4 demonstrates phantom acquisitions at b=5000s/m2. Even at this high b-value, artifacts in the uncorrected PEPTIDE acquisitions are subtle, with some small signal variations mitigated through the second-iteration reconstruction, suggesting successful estimation and correction of the eddy-currents. The estimated eddy-current phase appears approximately linear, and the associated field agrees closely with the FSL-EPI estimate obtained from 64 pairs of blipped-up and -down acquisitions.
While in-vivo data at b=5000s/mm2 has substantially lower-SNR than the phantom (Figure 5a), by using image phase in PEPTIDE rather than relying on small distortions in the magnitude images as in FSL-EPI, accurate eddy-field estimate is still feasible from a 3-direction dataset. Phantom (Figure 5b) and in-vivo (Figure 5c) PEPTIDE field estimates show good agreement with each other as well as with the FSL-EPI phantom estimates, while the in-vivo-FSL estimates contain significant deviation for this low-SNR, high-b-value acquisition.
Discussion/Conclusion
The time-resolved PEPTIDE technique has been demonstrated capable of accurately detecting eddy-current induced phase changes. Agreement is seen with the well-established FSL eddy technique in a high-SNR phantom, with PEPTIDE able to determine eddy-fields for arbitrary directions from as little as 3 diffusion-directions in-vivo at b=5000s/mm2. While the scanner data examined here appeared to have linear temporal field variations, based on simulations, this technique should also be able to detect higher-order temporal components, as well as higher-order spatial components, as needed. Future work would involve confirmation of the temporal field variations against field-probe measurements.Acknowledgements
This work was supported in part by the NIH R01EB020613, R01EB019437, R01MH116173, R01EB016695, P41EB030006, and U01-EB025162.References
1. Le Bihan, D., Poupon, C., Amadon, A., Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006 Sep;24(3):478-88.
2. Haselgrove, J.C., Moore, J.R. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med 1996;36:960 –964.
3. Jezzard, P., Barnett, A.S., Pierpaoli, C. Characterization of and correction for eddy current artifacts in echo planar diffusion imaging. Magn Reson Med 1998;39:801–812.
4. Calamante, F., Porter, D.A., Gadian, D.G., Connelly, A. Correction for eddy currents induced Bo shifts in diffusion-weighted echo-planar imaging. Magn Reson Med 1999;41:95–102.
5. Andersson JLR and Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125:1063-1078.
6. De Zanche, N., Barmet, C., Nordmeyer‐Massner J.A, and Pruessmann K.P. NMR probes for measuring magnetic fields and field dynamics in MR systems. Magn. Reson. Med. 2008, 60: 176-186.
7. Barmet, C., De Zanche, N., Wilm, B.J. and Pruessmann, K.P., A transmit/receive system for magnetic field monitoring of in vivo MRI. Magn. Reson. Med. 2009,62: 269-276.
8. Chan, R.W., von Deuster, C., Giese, D., et al., Characterization and correction of eddy-current artifacts in unipolar and bipolar diffusion sequences using magnetic field monitoring, Journal of Magnetic Resonance, 2014; 244:74-84.
9. Ma, R., Akçakaya, M., Moeller, S., Auerbach, E., Uğurbil, K., Van de Moortele, P.F. A field-monitoring-based approach for correcting eddy-current-induced artifacts of up to the 2nd spatial order in human-connectome-project-style multiband diffusion MRI experiment at 7T: A pilot study. Neuroimage. 2020;216:116861.
10. Wang, F., Dong, Z., Reese, T.G., et al. Echo planar time‐resolved imaging (EPTI). Magn Reson Med. 2019; 81: 3599– 3615.
11. Fair, M.J., Wang, F., Dong., Z., Reese, T.G., Setsompop, K. Propeller echo‐planar time‐resolved imaging with dynamic encoding (PEPTIDE). Magn Reson Med. 2020; 83: 2124– 2137.
12. Fair, M.J., Liao, C., Manhard, MK., Setsompop, K. Diffusion‐PEPTIDE: Distortion‐ and blurring‐free diffusion imaging with self‐navigated motion‐correction and relaxometry capabilities. Magn Reson Med. 2020; 00: 1–17.
13. Dong, Z., Wang, F., Reese, T.G., et al. Tilted‐CAIPI for highly accelerated distortion‐free EPI with point spread function (PSF) encoding. Magn. Reson. Med. 2019; 81: 377– 392.
14. Andersson JLR, Skare S and Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20:870-888, 2003.
Figures