0618
Fast T2-mapping in prostate cancer based on echo-time domain compressed sensing1Philips Research, Hamburg, Germany, 2Department of Radiation Oncology, The Netherlands Cancer Institute, Amsterdam, Netherlands, 3Philips Healthcare, Best, Netherlands, 4Department of Medical Physics, The Netherlands Cancer Institute, Amsterdam, Netherlands
Synopsis
T2w-MRI plays an important role in prostate cancer providing information on the location and aggressiveness in diagnosis and therapy. T2-mapping may provide objective characterization, but is hampered by long acquisition time, which has been addressed by dedicated acceleration techniques (e.g. k-t T2-mapping). We investigated T2-mapping in a prostate cancer patient based on a 4-minute protocol with Poisson-disk prospective irregular sub-sampling in the ky-TE domain in combination with a low rank and sparsity constraint compressed sensing reconstruction. The regularization parameters were investigated, and compressed sensing results were compared to separately acquired k-t T2-maps with respect to quality and noise.
Introduction
T2-weighted MRI plays an important role in prostate cancer (PCa) providing information on the location, volume and grade in diagnosis/therapy planning or active surveillance. However, the qualitative nature of these images complicates the objective comparison between patients as well as the quantification of treatment response. To address this, T2-mapping is explored for objective PCa characterization1 and monitoring2. Non-accelerated multi-echo spin-echo (MESE) T2-mapping with good coverage and high resolution (≤1mm) is prohibitively slow. Previously, k-t T23 was proposed for T2-mapping and successfully applied in patient studies4, achieving a scan time of 4½ minutes (60 mm coverage FH, 12 echoes). Here, aliasing artifacts are reduced by trained kernels in ky-TE domain. Mono-exponential decay is assumed, and regular sub-sampling is required, which may bias T2-values and limit acceleration. Thus, T2-mapping using compressed sensing over echo times as extra dimension (T2-CSXD) was proposed5. The prior knowledge used in T2-CSXD is more generic, potentially enabling higher acceleration with irregular sub-sampling. In this study, we directly compare T2-CSXD, using various regularization settings, with k-t T2 in prostate tissue for identical resolution and scan time, including short-T2 cancerous areas.Methods
Details on T2-CSXD mapping are provided in reference 5. In short, irregular sub-sampling along the TE dimension was implemented by introducing ky blip gradients during a MESE readout. Reconstruction used a fast low-rank and sparsity regularization algorithm6, solving the following minimization problem for N echoes:$$\underset{M}{arg\ min}=(\frac{1}{2}\| EM-P \|^2_2+\lambda_1 \| W(M) \|_1+\lambda_2 \| M \|_*)$$,
where M is a matrix with N columns of vectorized echo images, P a matrix of N measured k-spaces, E the encoding matrix, including sub-sampling and coil sensitivity maps, W the wavelet transform, $$$\| \|_*$$$ the nuclear norm. Regularization parameters were varied, 0≤λ1≤0.6, 0≤λ2≤0.003, to investigate regularization dependent image quality, T2-bias and standard deviation (SD). Reconstruction was implemented within the MR software but run on a workstation for different regularizations, with standard maximum-likelihood fitting to generate T2-maps from the echo series. A PCa patient was scanned with both k-t T2 and the proposed T2-CSXD technique on a 3T MRI system (Ingenia, Philips, NL) with informed consent, protocol approved by the institutional review board. k-t T2 and T2-CSXD: FOV = 170x170x60mm3, voxel size =1x1x3 mm3, TR = 5000 ms, 12 echoes, TEn = (32 + n*16) ms, α=90°, refocusing control 120°, B1max=9.5μT. For T2-CSXD: Acceleration R=10, irregular Poisson-disk sampling in ky-TE, 7 central fully sampled lines, acquisition time 4:10 min. For k-t-T2: Partial-Fourier factor 0.6, R=8 (2×SENSE, 4×ky-TE sub-sampling), 4 autocalibration lines, kernel 3×3×9 (M, P, echo), acquisition time 4:17 min. To compare the T2-maps, three single slice ROIs were delineated (diameter of 3mm) in regions within the prostate with low (83ms), intermediate (145ms) and high (220ms) mean T2-values (<T2>). The relative difference ΔT2[%] of <T2> between k-t T2 and T2-CSXD and the relative SD[%] for T2-CSXD were investigated as a function of the regularization parameters.
Results
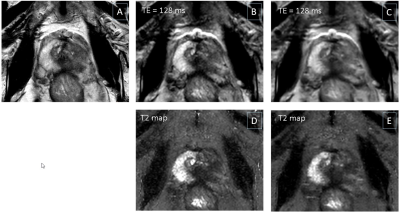
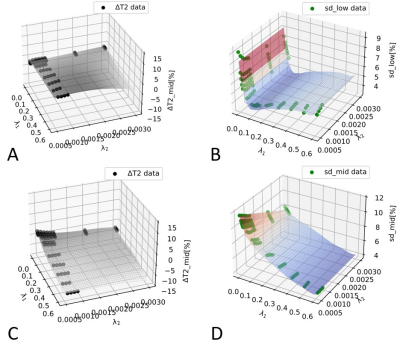
Fig.1 shows example slices from a PCa patient, including a T2w anatomical image (Fig.1A), k-t T2 results (Fig.1B,D) and T2-CSXD results (Fig.1C,E) with regularization λ1=0.02/λ2=0.0007. Single echo images at TE=128ms (Fig.1D,E) show a good image quality for both methods, with slightly less noisy appearance of T2-CSXD. The T2 map quality appears improved with less apparent noise for T2-CSXD. This is confirmed by lower SD-values found in the ROI analysis for T2-CSXD, particularly for low <T2> (SD<5% for 83ms for a large range of regularizations), in comparison to k-t-T2 (SD=13±0.5% for all ROIs/<T2>). Fig.2 represents the regularization parameter assessment, using (λ1, λ2) as (x,y) coordinates and measured ΔT2[%] (Fig.2A,C) or SD (Fig.2B,D) as z-coordinate in a 3D plot. A smoothed-spline surface highlights the trends. Data for low <T2> (Fig.2A,B) and intermediate <T2> (Fig.2C,D) are shown. For short <T2>, SD reaches a minimum at λ1=0.08 (Fig.2B), while for mid-range <T2>, SD is observed to further decrease for larger λ1 (Fig.2D). For large <T2> (data not shown), a minimum is found for small λ1<0.1. T2-CSXD values are in general a few percent lower than those obtained by k-t T2 and decrease further with increasing λ1 and λ2.Discussion & Conclusion
T2-CSXD showed distinct surface patterns in (λ1, λ2) for different T2-ranges, which guides regularization for specific acquisition parameters (e.g., Nechoes). Without regularization, sub-sampling artefacts and noise-amplification are expected, leading to a high SD (noise). With increased λ1, reconstruction is stabilized, and artefacts/noise reduced, explaining the observed SD decrease. SD-increase for large λ1 is expected due to wavelet artifacts. Short-T2 areas (low SNR) are expected to be more affected by wavelet artifacts (faster SD-rise with λ1). Increased bias in T2 is expected at large λ1/λ2 as reconstruction relies increasingly on prior knowledge introduced by the regularization and less on measured data. As a limitation, T2-bias is also expected for k-t-T2 such that no absolute reference is available. Short T2<100ms are typical for tumor tissue at 3T, reliably detected using 0<λ1<0.1 and λ2<0.001, according to our data. As regularization-dependent quality is varying for T2-value ranges, a compromise for relevant T2-values in PCa (80-150 ms) can be established. In conclusion, T2-maps using 10× acceleration were obtained in a PCa patient with promising quality and within clinically relevant acquisition time, using a T2 CS technique with fine-tuned regularization.Acknowledgements
No acknowledgement found.References
1. Chatterjee A, Devaraj A, Mathew M, Szasz T, Antic T, Karczmar GS, Oto A. Performance of T2 Maps in the Detection of Prostate Cancer. Acad Radiol. 2019 Jan;26(1):15-21.
2. Foltz WD, Wu A, Chung P, Catton C, Bayley A, Milosevic M, Bristow R, Warde P, Simeonov A, Jaffray DA, Haider MA, Ménard C. Changes in apparent diffusion coefficient and T2 relaxation during radiotherapy for prostate cancer. J Magn Reson Imaging. 2013 Apr;37(4):909-16.
3. Liu W, Turkbey B, Sénégas J, Remmele S, Xu S, Kruecker J, Bernardo M, Wood BJ, Pinto PA, Choyke PL. Accelerated T2-mapping for characterization of prostate cancer. Magn Reson Med. 2011 May;65(5):1400-6.
4. van Houdt PJ, Agarwal HK, van Buuren LD, Heijmink SWTPJ, Haack S, van der Poel HG, Ghobadi G, Pos FJ, Peeters JM, Choyke PL, van der Heide UA. Performance of a fast and high-resolution multi-echo spin-echo sequence for prostate T2-mapping across multiple systems. Magn Reson Med. 2018 Mar;79(3):1586-1594.
5. Keupp J, Doneva M, Meineke J, and Forthmann P. Proc. Intl. Soc. Mag. Reson. Med. 28 (2020) 888
6. J. Yao, Z. Xu, X. Huang, and J. Huang, “An efficient algorithm for dynamic MRI using low-rank and total variation regularizations,” Med. Image Anal., vol. 44, pp. 14–27, Feb. 2018.
Figures