0617
Multiband Multitasking for Cardiac T1 Mapping1UIH America, Inc., Houston, TX, United States, 2United Imaging Healthcare, Shanghai, China
Synopsis
Multiband (MB) technique is combined with multitasking for increased spatial coverage of the heart without prolonging scan time. Two different MB multitasking implementations were developed and compared with the conventional multitasking technique on volunteers and phantoms. Both methods demonstrated similar capabilities in solving multiple ‘tasks’ when compared with the reference method and exhibited good agreement in T1 mapping values. MB multitasking is a promising technique for cardiac MR.
Introduction
Quantitative T1 mapping of the heart is considered an important biomarker for various diseases [1]. T1 cardiovascular magnetic resonance (CMR) multitasking is a free-breathing, ECG-free technique featuring high spatial and temporal resolution myocardial T1 mapping, by utilizing low-rank tensor imaging model to solve multiple ‘tasks’ at the same time [2,3]. Despite its ability to produce 2D T1 cine maps in a short 1-min scan, T1 CMR multitasking is capped at 50% efficiency due to scan time spent on navigator readouts that had no contribution to spatial coefficient maps. Navigator lines of a constant angle interleaved between golden-angle radial imaging lines are needed for temporal subspace estimation. To further improve its scan efficiency, T1 CMR multitasking is combined with multiband (MB) technique [4] for accelerated T1 mapping of the heart under free-breathing and without ECG. Two different MB multitasking strategies are proposed and validated on phantom and volunteers.Methods
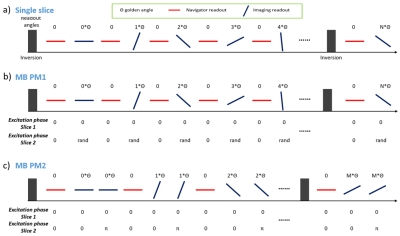
Sequence design: A pulse sequence based on 2D radial spoiled-GRE was developed following a previous application [2], and subsequently modified to excite multiple slices simultaneously (Figure 1). A MB factor of two was used in the study as a proof-of-concept although the principals are applicable to higher acceleration. Excitation phases of the imaged slices varied throughout data acquisition. Two different phase modulation (PM) strategies were proposed and dubbed ‘PM1’ and ‘PM2’ accordingly. Signal of slice 2 was modulated by a random excitation phase at imaging readout in PM1, while that in PM2 was modulated by alternating phases of 0 and π resulting in two consecutive imaging readouts. In contrast to slice 2, signal of slice 1 experienced no modulation.Reconstruction: The underlying multidimensional images of the j-th slice was represented by a 4-way tensor $$$\tt A^{j}$$$ with its first dimension concatenating all voxel locations and other dimensions indexing cardiac-motion, respiratory-motion, and inversion recovery. Because both slices are acquired simultaneously and are from the same imaged object, a shared multi-dimensional temporal basis $$$\Phi$$$ was assumed and was determined from navigator readouts. Using the principle of partial separability, $$$A_\left(1\right)^j=U^{j}\Phi$$$, where $$$A_\left(1\right)^j$$$ is the mode-1 matricization of $$$\tt A^{j}$$$ and $$$U^{j}$$$ is the spatial factors for the j-th slice, $$$U^{j}$$$ can be recovered by solving the optimization problem: $$\widehat{U^{j}}=argmin_{U^{j}}||d_{img}-\Omega F \sum_{j=1}^2S^{j}P^{j}U^{j}\Phi||_2^2+\sum_{j=1}^2R(U^{j})$$
where $$$d_{img}$$$ is the acquired imaging readout, $$$\Omega$$$ is undersampling operator, $$$F$$$ is Fourier transform operator, $$$S^{j}$$$ is the coil sensitivity operator for the j-th slice, $$$P^{j}$$$ is the phase modulation operator for the j-th slice, and $$$R$$$ is spatial regularization operator.
$$$P^{j}$$$ is created by MB phase modulation and can be expressed as:$$P^{j}=e^{i\theta^{j}}$$
where $$$\theta^{j}$$$ are the excitation phases for the j-th slice at imaging readout. Note that for MB-PM1 and PM2 we have $$$\theta^{1}=0$$$. For MB-PM2 we have $$$\theta^{2}=e^{i (m-1)\pi }$$$ for the m-th imaging readout.
The remaining reconstruction steps follow the conventional multitasking reconstruction pipeline. In MB-PM2 reconstruction the two neighboring imaging readouts are first decoupled by addition and subtraction as in autocalibrated multiband imaging [5-7], then signal of two slices are separately handled for faster reconstruction.
Study experiment: All data were acquired on a clinical 3T scanner (uMR790, United Imaging Healthcare, Shanghai, China). Imaging parameters are summarized in Table 1. The same imaging parameters were used regardless of techniques, meaning that MB techniques have twice the coverage with the same scan time. ISMRM/NIST phantom [8] was used to assess the accuracy of T1 quantification. Five volunteers were recruited after IRB consent for short-axis cardiac imaging with MB multitasking. Two separate single-slice multitasking scans were performed at 9 matching slice positions and served as a reference for comparison. The average myocardium T1 values at systole and diastole of each slice were measured and analyzed using statistics.
Results
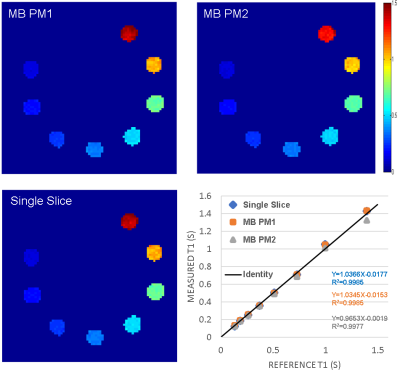
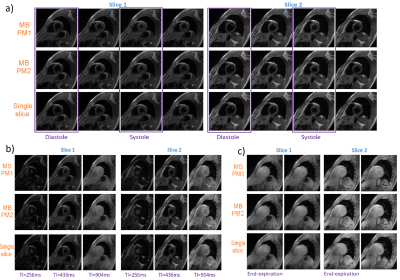
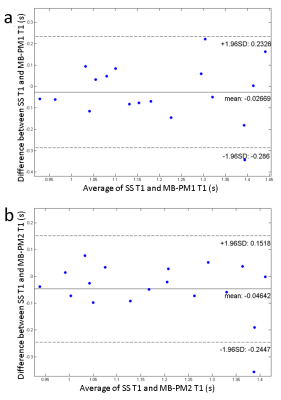
Phantom T1 maps are shown in Figure 2. Both MB-PM1 and MB-PM2 demonstrate excellent correlation ($$$R^{2}$$$>0.997) with true values as reported in the phantom’s manual. Figure 3 illustrates typical volunteer images reconstructed by all techniques at matching slice positions, along different dimensions. MB multitasking clearly depicts changes along respective dimensions when compared to single-slice multitasking. Figure 4 shows typical diastole and systole T1 maps at matching slice positions. Bland-Altman plots (Figure 5) of myocardium T1s of both MB strategies demonstrate good agreement with that of single-slice. No statistical differences are found at 0.05 significance level between T1s of MB-PM1 and single-slice (p=0.404), and between MB-PM2 and single-slice (p=0.068).Conclusion and Discussion
The feasibility of MB multitasking was demonstrated, with two different strategies to achieve MB encoding. Both methods demonstrated good agreement with the reference single-slice technique, as illustrated by volunteer and phantom results. MB-PM2 was slightly inferior to MB-PM1 in T1 quantification as indicated by the lower correlation coefficients in the phantom study and the lower p value in the volunteer study. This might be caused by temporal blurring when adding and subtracting neighboring readouts. MB multitasking has the potential to increase CMR multitasking spatial coverage without prolonging scan time. Further validation in a larger patient cohort is warranted.Acknowledgements
This work was partially facilitated by a non-exclusive license agreement between Cedars-Sinai Medical Center and United Imaging Healthcare.References
[1] Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014 Jan 4;16(1):2.
[2] Shaw JL, Yang Q, Zhou Z, Deng Z, Nguyen C, Li D, Christodoulou AG. Free-breathing, non-ECG, continuous myocardial T1 mapping with cardiovascular magnetic resonance multitasking. Magn Reson Med. 2019 Apr;81(4):2450-2463.
[3] Christodoulou, A.G., Shaw, J.L., Nguyen, C. et al. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng 2, 215–226 (2018).
[4] Breuer FA, et al., Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging, Magn Reson Med. 2005 Mar;53(3):684-91.
[5] Ferrazzi G, et al., Autocalibrated multiband CAIPIRINHA with through-time encoding: Proof of principle and application to cardiac tissue phase mapping, Magn Reson Med. 2019 Feb;81(2):1016-1030.
[6] Ding et. al. Highly Accelerated Free Breathing Whole Heart Real-time Cine using AuTO-calibrated Multiband Imaging and Compressed Sensing (ATOMICS), ISMRM 2020: 3463
[7] Zheng et. al. Auto-calibrated simultaneous multi-slice cardiac bSSFP Cine MRI using gradient temporal modulation, ISMRM 2020: 2176
[8] Kathryn E.K., Karl F.S., Michael A.B., et al. Multi-site, multi-vendor comparison of T1 measurement using ISMRM/NIST system phantom, ISMRM 2016: 3290
Figures