0606
Identification of diffusion-based micro-structural measures most sensitive to multiple sclerosis focal damage using GAMER-MRI1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 4Division of Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 5Department of Computer Science, University of Verona, Verona, Italy, 6Signal Processing Laboratory (LTS5), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 7Medical Image Analysis Laboratory, Center for Biomedical Imaging (CIBM), University of Lausanne, Lausanne, Switzerland, 8Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 9Center for medical Image Analysis & Navigation, Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
We applied an attention-based convolutional neural network to select discriminating diffusion measures derived from mathematical models of multi-shell diffusion data in the classification of multiple sclerosis lesions. Further, we correlated the selected measures or their combinations with the Expanded Disability Status Scale (EDSS) and the serum level of neurofilament light chain (sNfL). Our results show that the combinations have stronger correlations with EDSS and sNfL than the individual measures. The proposed method might be useful for selecting the microstructural measures most discriminative of focal tissue damage and identifying the combination most related to clinical disability and neuroaxonal damage.
Introduction
Multi-shell diffusion-weighted imaging (mDWI) may probe microstructural tissue damage and repair in multiple sclerosis (MS) patients1,2. From mDWI, biophysical microstructure models can be fitted to measure different water compartments within the brain tissue. However, different models measure the same compartment differently due to their different biophysical assumptions. Therefore, selecting the most discriminating quantitative diffusion measures (qDMs) for a given neurological disease remains challenging. In our previous work3, we developed and validated an attention-based convolutional neural network – GAMER-MRI – to rank the importance of the input quantitative MRI contrasts using attention weights (AWs) in the classification of MS lesions. Here, we further developed the method to select discriminating inter-correlated qDMs in the classification of MS lesions and perilesional tissue (PeriT). Furthermore, we explored the relationship between the selected qDM, or their combinations, with the Expanded Disability Status Scale (EDSS) and the neurofilament light chain in the serum (sNfL), which are respectively (i) a clinical measure of disability in MS patients and (ii) a biological measure of neuroaxonal damage4,5.Methods
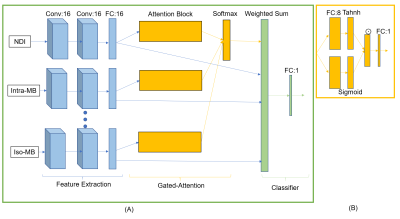
One-hundred-and-twenty-three MS patients (84 relapsing-remitting and 39 progressive, 71 females, age range=44.7±14.0, median EDSS=2.5, EDSS range 0.0-8.0) underwent MRI on a 3T whole-body MR system (Siemens MAGNETOM Prisma). The protocol included: 1mm3 isotropic 3D FLAIR and 1.8mm3 isotropic mDWI (TR/TE:4500/75ms) with b-values 0/700/1000/2000/3000s/mm2 and 137 directions split among them. Twelve qDMs for the isotropic and intra-axonal compartments were reconstructed from seven models, including Ball and Stick6, NODDI7, SMT-NODDI8, Microstructure Bayesian approach (MB)9, MCMDI10, NODDIDA11, DIAMOND12 and microstructure fingerprinting13. The qDMs were masked by the brain mask and subject-wise normalized. Among 123 patients, 84 patients were used in a 5-fold cross-validation. The other 39 patients were randomly selected into a pure test dataset. White matter lesions (WMLs) were automatically segmented14 and manually corrected on FLAIR. The PeriT was defined as WM tissue locating within a 3-voxel region around the lesions. In the end, 1,402 WML patches and 1,665 PeriT patches were in the test dataset, and 4,409 WML patches and 5,289 PeriT patches were in the cross-validation dataset.GAMER-MRI consisted of feature extraction, gated attention mechanism (GAM)15 and classification3 (Fig. 2). The hyperparameters included the number of the convolutional filters, of neurons for the hidden feature and of neurons in the layers in the GAM. They were 16, 16 and 8, respectively. The weighted sampler and the cross-entropy loss function were used. The batch size was 256. The evaluation metric was the area under the receiver operating characteristic curve (AUC). To avoid overfitting, data augmentation, the learning-rate-reduce-on-plateau scheduler and AdamW16 (a regularized optimizer) were used. Intrinsic strong correlation between the qDMs can lead to instability of the obtained AWs and the ranked order. Therefore, to avoid determination solely based on the AWs, the selection of discriminating qDMs was an iterative process. It started from the qDM whose AW was dominant in the validation datasets in all the cross-validation folds. If no qDM was selected, the qDMs whose AWs were ranked 1st or 2nd in all the folds were selected. The iterative selection stopped when the sum of their AWs was over 0.5, which meant that the selected measures were more important than 50% of the input qDMs.
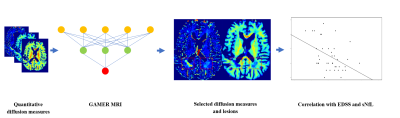
To assess which selected subject-wise normalized qDMs, or their combination, was best correlated with patients’ EDSS as well as sNfL in the test dataset, we first averaged the qDMs within each lesion and then over lesions within each patient. In 31/39 patients of the test dataset, we quantified sNfL. Then, we performed Spearman’s correlation with two-sided 20,000 permutation tests. The Benjamin-Hochberg procedure17 was performed to control the false discovery rate (FDR) with the threshold 0.05. The flowchart is shown in Fig. 1.
Results and Discussion
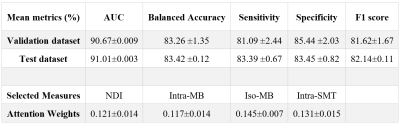
In Table 1, we report the average performance on the (i) validation dataset over 5-fold cross-validation and (ii) on the independent test dataset. The evaluation metrics indicated that GAMER-MRI learned pivotal information for the target classification. As expected, because of the highly correlated nature of the studied diffusion-based measures, the difference among the obtained AWs was small and their ranking was fluctuating. This was alleviated by the proposed selection process. The qDMs selected by using the validation datasets were the neurite density index (NDI) from NODDI, the intra-axonal and isotropic compartment from MB (Intra-MB and Iso-MB) and the intra-axonal compartment from SMT-NODDI (Inra-SMT). Their average AWs of the corrected predicted samples are also reported in Table 1.The correlation coefficients (ρ) and the corresponding p-values of EDSS and the selected normalized qDM, their statistically significant combinations and conventional lesion load are in Table 2. The correlation with sNfL is in Table 3. The sum of measures quantifying intra-axonal and isotropic diffusion was best correlated with disability, even stronger than those qDMs alone or even conventional MRI lesion load.
Conclusions
In summary, our work showed that the proposed attention-based neural network and the selection process can select important qDMs, despite they being highly inter-correlated. Those measures can potentially be combined to enhance the correlation with the clinical measures. Future work will be required to directly find the best combinations without using a statistical test and to better interpret their pathological meaning.Acknowledgements
This research is supported by Swiss National Funds PZ00P3_154508, PZ00P3_131914 and PP00P3_176984 and we thank all the patientsfor their participation.References
1. Schneider, T. et al. Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: A pilot study. Funct. Neurol. (2017) doi:10.11138/FNeur/2017.32.2.097.
2. Lakhani, D. A., Schilling, K. G., Xu, J. & Bagnato, F. Advanced multicompartment diffusion MRI models and their application in multiple sclerosis. American Journal of Neuroradiology (2020) doi:10.3174/AJNR.A6484.
3. Lu, P.-J. et al. GAMER MRI: Gated-Attention MEchanism Ranking of multi-contrast MRI in brain pathology. NeuroImage Clin. 102522 (2020) doi:10.1016/j.nicl.2020.102522.
4. Siller, N. et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult. Scler. J. (2019) doi:10.1177/1352458518765666.
5. Barro, C. et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain (2018) doi:10.1093/brain/awy154.
6. Behrens, T. E. J. et al. Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging. Magn. Reson. Med. (2003) doi:10.1002/mrm.10609.
7. Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–16 (2012).
8. Cabeen, R. P., Sepehrband, F. & Toga, A. W. Rapid and Accurate NODDI Parameter Estimation with the Spherical Mean Technique. in ISMRM (2019).
9. Reisert, M., Kellner, E., Dhital, B., Hennig, J. & Kiselev, V. G. Disentangling micro from mesostructure by diffusion MRI: A Bayesian approach. Neuroimage (2017) doi:10.1016/j.neuroimage.2016.09.058.
10. Kaden, E., Kelm, N. D., Carson, R. P., Does, M. D. & Alexander, D. C. Multi-compartment microscopic diffusion imaging. Neuroimage (2016) doi:10.1016/j.neuroimage.2016.06.002.
11. Jelescu, I. O. et al. One diffusion acquisition and different white matter models: How does microstructure change in human early development based on WMTI and NODDI? Neuroimage (2015) doi:10.1016/j.neuroimage.2014.12.009.
12. Scherrer, B. et al. Characterizing brain tissue by assessment of the distribution of anisotropic microstructural environments in diffusion-compartment imaging (DIAMOND). Magn. Reson. Med. (2016) doi:10.1002/mrm.25912.
13. Rensonnet, G. et al. Towards microstructure fingerprinting: Estimation of tissue properties from a dictionary of Monte Carlo diffusion MRI simulations. Neuroimage (2019) doi:10.1016/j.neuroimage.2018.09.076.
14. La Rosa, F. et al. Multiple sclerosis cortical and WM lesion segmentation at 3T MRI: a deep learning method based on FLAIR and MP2RAGE. NeuroImage Clin. (2020) doi:10.1016/j.nicl.2020.102335.
15. Ilse, M., Tomczak, J. M. & Welling, M. Attention-based Deep Multiple Instance Learning. (2018).
16. Loshchilov, I. & Hutter, F. Decoupled weight decay regularization. in 7th International Conference on Learning Representations, ICLR 2019 (2019).
17. Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (1995) doi:10.1111/j.2517-6161.1995.tb02031.x.
Figures