0596
Optimized 3D spiral ultra-short echo time free-breathing pulmonary imaging on a high-performance low-field 0.55T scanner1Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Pulmonary Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Siemens Medical Solutions USA Inc., Malvern, PA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, MD, United States
Synopsis
Low signal in the lung parenchyma, and reduced signal-to-noise at 0.55T makes high resolution lung imaging challenging. SNR can be improved by using longer spiral readouts. However, these readouts are susceptible to artifacts from trajectory errors, and blurring from concomitant fields which are amplified at lower field strengths. Here we present an optimized self-gated, ultra-short echo time, stack-of-spirals acquisition which leverages inline corrections for trajectory imperfections, measurement drift, and concomitant fields to enable robust high resolution lung imaging on low field scanners. We also demonstrate the improvement in image quality in patients with lung nodules and Lymphangioleiomyomatosis (LAM).
Introduction
High-performance low field systems are well suited for structural lung imaging due to reduced susceptibility and prolonged T2* times1. Ultra-short echo time (UTE) sequences are important for high-resolution structural imaging of lungs at clinical field strengths2,3 but require dedicated optimization for low-field systems. Low SNR is a major limitation of low-field systems, which can be mitigated using longer readouts to improve SNR-efficiency. This can be accomplished with spiral-readouts, but these readouts are susceptible to off-resonance and concomitant field related blurring. Off-resonance blurring scales with B0 and is therefore reduced at lower field strengths; however, concomitant field artifacts scale inversely with B0 and are amplified at lower field strengths4.Our aim is to develop a robust method for free-breathing, high-resolution pulmonary imaging at 0.55T . In this work, we optimized a self-gated stack-of-spirals UTE sequence and leverage corrections for trajectory errors, measurement drift and concomitant fields to mitigate artifacts, with corrections implemented inline for rapid online image reconstruction5.
Methods
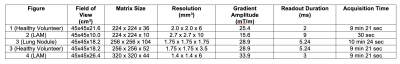
Data Acquisition: A prototype free-breathing 3D T1-weighted ultra-short echo time (UTE) spoiled gradient echo stack-of-spirals sequence was used for imaging on a 0.55T MRI system (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Additional readouts were acquired along the superior-inferior (SI) direction to estimate the respiratory signal with temporal resolution of <300ms. All scans used golden angle increments of spiral interleaves. Other image parameters are shown in Table 1.Reconstruction: Respiratory waveforms were extracted from the SI readouts using previously published methods6. An additional correction for drift in the respiratory navigator signal was implemented. Data was retrospectively binned before reconstruction to reduce respiratory motion artifacts. The threshold for binning was empirically chosen to be 40% of the total data at the most stable respiratory phase.
Data was reconstructed inline using Gadgetron5 on a system equipped with Dual Intel Xeon processors, 512 GB RAM, 3x Nvidia Quadro RTX 8000 GPUs. Density compensation weights were estimated using an iterative calculation7. We used a conjugate gradient SENSE reconstruction with 3 iterations to reduce artifacts from non-uniform under sampling in k-space for binned FB data.
Spiral trajectory and concomitant field corrections: Inaccuracies in spiral trajectories were corrected using gradient system impulse response functions8. The local phase caused by concomitant fields was estimated for each spiral acquisition. Corrections were applied using multi-frequency interpolation, whereby each spiral acquisition was demodulated at 10-35 frequencies and a pixel-wise linear combination of the images was used to estimate the corrected image9.
Patient imaging: Human subjects imaging was performed with permission from the local Institutional Review Board. We demonstrate high-resolution UTE imaging in two healthy volunteers, two patients with lymphangioleiomyomatosis (LAM), a rare cystic lung disease, and one patient with lung nodules. Images were acquired in both coronal and axial views (parameters shown in Table 1 to demonstrate artifacts due to concomitant fields.
Results
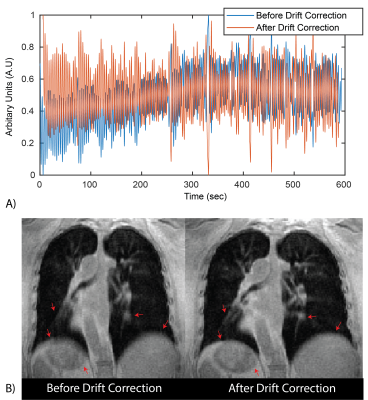
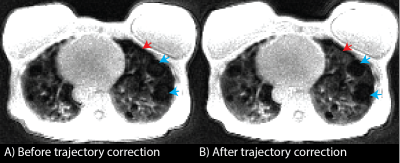
All reconstructions were performed in Gadgetron with reconstruction times < 5 min. Figure 1 shows an example of the self-gated respiratory navigator signal before and after drift correction. This drift is likely due to hardware heating from this gradient-heavy sequence. The resulting images demonstrate reduced respiratory motion artifacts after drift correction, especially noticeable around the liver dome and blood vessels.Figure 2. demonstrates the improvement in image quality following correction of trajectory imperfections in a patient with LAM. Trajectory correction improves signal intensity in the lung parenchyma which makes it possible to visualize cysts in the LAM patient which were not visible before trajectory correction. It also corrects signal intensity artifacts and reduces the signal that sometimes appears inside cysts which drastically improves visibility of cysts.
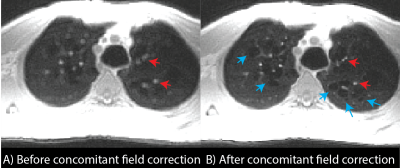
Improvements in image quality from concomitant field correction and respiratory binning are demonstrated in Figures 3 and 4 for coronal and axial orientations. In Figure 3, blue arrows show improvements in image sharpness with respiratory binning and red arrows show improvements in sharpness from concomitant field corrections. The 7.5 mm lung nodule can be clearly visualized using 1.75mm isotropic resolution. In Figure 4, concomitant field corrections in the axial orientation demonstrate the improvements in image quality for patient with LAM. Improvements in the appearance of cyst boundaries (blue arrows) and vessel sharpness (red arrows) are shown. Concomitant field correction significantly improves the visibility of cysts and image sharpness in these subjects.
Discussion and Conclusion
We have presented an optimized implementation of free-breathing 3D stack-of-spirals UTE pulmonary imaging sequence for a high-performance 0.55T system with fast inline reconstruction. We demonstrate that a combination of robust respiratory binning, drift correction, trajectory corrections, and concomitant field corrections are necessary to achieve diagnostic image quality for high-resolution UTE imaging at 0.55T. In particular, concomitant fields, which scale inversely with B0, require additional attention when combining high-amplitude gradients with lower field strengths. Using our optimized method, we were able to achieve high-quality structural lung imaging with a resolution of down-to 1.75mm isotropic.Acknowledgements
The authors thank Kendall O’Brien and Christine Mancini for their imaging expertise and Margaret (Peg) Lowery, Jennifer Henry, Amelia Nargozian, Patricia Julien-Williams and Amanda Jones for assistance with patient recruitment. The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. The study was supported in part by the Intramural Research Program, NIH/NHLBI.References
1. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. 2019;293(2):384-393.
2. Mugler JP, Meyer CH, Pfeuffer J, Stemmer A, Kiefer B. Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging. In: International Society of Magnetic Resonance in Medicine. Vol 11. Honululu; 2017:4-6.
3. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70(5):1241-1250.
4. King KF, Ganin A, Zhou XJ, Bernstein MA. Concomitant gradient field effects in spiral scans. Magn Reson Med. 1999;41(1):103-112.
5. Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med. 2013;69(6):1768-1776.
6. Grimm R, Bauer S, Kiefer B, Hornegger J, Block T. Optimal Channel Selection for Respiratory Self-Gating Signals. In: International Society of Magnetic Resonance in Medicine. Salt Lake City; 2013:3749.
7. Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn Reson Med. 1999;41(1):179-186.
8. Vannesjo SJ, Graedel NN, Kasper L, et al. Image reconstruction using a gradient impulse response model for trajectory prediction. Magn Reson Med. 2016;76(1):45-58.
9. Man LC, Pauly JM, Macovski A. Multifrequency interpolation for fast off-resonance correction. Magn Reson Med. 1997;37(5):785-792.
Figures