0591
Response of Hyperpolarized 129Xe MRI measures of ventilation and gas-exchange to anti-fibrotic treatment in Idiopathic Pulmonary Fibrosis1Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Medicine, University of Wisconsin - Madison, Madison, WI, United States, 4Radiology, Duke University, Durham, NC, United States, 5Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Predicting outcomes and monitoring longitudinal treatment response in IPF is unreliable using currently available clinical biomarkers. We investigate associations between hyperpolarized 129Xe MRI biomarkers of ventilation and gas exchange and treatment with anti-fibrotic medication in IPF patients over a 1-year period. Anti-fibrotic treatment was associated with improved ventilation and gas exchange, relative to no anti-fibrotic treatment, after 1 year. Within-patient improvements in gas exchange were significantly larger in patients treated with anti-fibrotic medications. No longitudinal associations were found between anti-fibrotic treatment and spirometry, suggesting the imaging biomarkers may be more useful for monitoring anti-fibrotic treatment response in IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease with generally poor prognosis, and available clinical biomarkers are currently insufficient for reliable prediction of disease progression and patient outcomes1. The use of hyperpolarized (HP) 129Xe MRI to characterize IPF has been a recent focus in the literature2, however the utility of these imaging biomarkers for longitudinal monitoring of disease is not well established. Recent work demonstrates that two HP 129Xe imaging biomarkers of ventilation and gas exchange, specifically high ventilation percent (HVP) and red blood cell to tissue barrier (RBC:Barrier) ratio, are significant baseline predictors of IPF disease progression3,4. Here, we investigate how these metrics respond longitudinally to targeted IPF anti-fibrotic (AF) medication therapy5.Methods
IPF patients (N=14, 13M:1F, age 69.1±9.8 years) were prospectively recruited and underwent both ventilation and dissolved-phase 129Xe MRI at baseline and at a 1-year follow-up timepoint. Same day pulmonary function tests were also performed at most time points, including forced vital capacity percent predicted (FVC%p) and diffusing capacity of the lung for carbon monoxide (DLCO%p). One patient did not have a DLCO measurement at baseline, and 4 did not have a DLCO measurement at follow-up. Additionally, 1 patient did not have an FVC measurement at follow-up. A subset of patients (N=8, 7M:1F, age 66±8.4 years) were clinically prescribed either Pirfenidone (N=7) or Nintedanib (N=1) for treatment of IPF5 (the “Anti-fibrotic” or AF group). The remaining patients (N=6, 6M, age 73.3±10.6 years) were not prescribed anti-fibrotic IPF medication (the “No anti-fibrotic” or no-AF group). Ventilation data from HP 129Xe MRI was analyzed using semi-automatic k-means clustering, where signal intensity was classified into 4 levels and quantified as the corresponding percent of lung volume, resulting in ventilation defect percent (VDP), low ventilation percent (LVP), medium ventilation percent (MVP), and high ventilation percent (HVP)6. Dissolved-phase HP 129Xe was separated into red blood cell (RBC) and parenchymal tissue compartments (i.e. gas exchange “Barrier”) using spectroscopic imaging with a 1-point Dixon technique7, and the average RBC:Barrier ratio over the whole lung was computed. Statistical comparisons between medication groups were performed using t-tests and were considered significant when P<0.05.Results
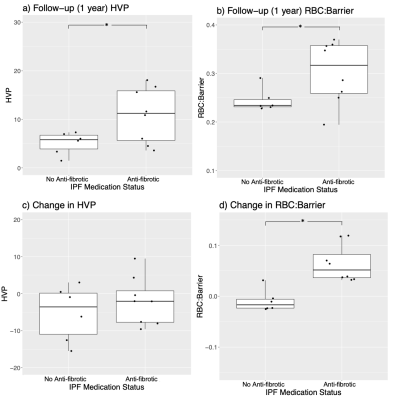
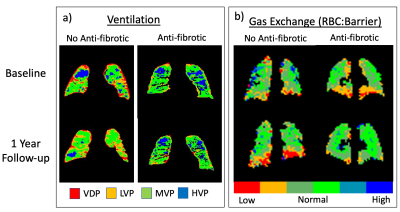
At baseline, FVC%p (P=0.026) was lower in the group receiving AF therapy, but there was no difference between groups for DLCO%p, HVP or RBC:Barrier (Table 1). At the 1 year follow-up FVC%p was similar to baseline (P=0.025), while both HVP and RBC:Barrier were significantly higher for the group receiving AF medication (HVP, P=0.027, RBC:Barrier, P=0.041). Boxplots of the MRI data are shown in Figure 1a and 1b. Within-patient increase in the RBC:Barrier ratio over the 1-year time period was significantly different between medication groups (P=0.0006) but not for HVP, shown in Figure 1c and 1d. Neither FVC%p or DLCO%p changed significantly between time points for the two medication groups (Table 1). Figure 2 shows example parametric maps of HVP and RBC:Barrier in a patient treated with Pirfenidone and a patient not taking AF medication for IPF. In the patient not on AF medication, the reduction in HVP and decline in RBC:Barrier ratio after 1 year is apparent compared to the patient taking Pirfenidone where HVP is maintained and RBC:Barrier recovers over the same time period.Discussion
In this small imaging study, administration of AF medication for IPF did not have a significant impact on spirometric measures typically used to monitor disease progression in IPF, specifically FVC%p and DLCO%p. However, HP 129Xe MRI measures of ventilation (HVP) and gas exchange (RBC:Barrier) showed a significant change (improvement) after medication. Patients taking AF medication had both significantly higher HVP and RBC:Barrier relative to no-AF patients after 1 year, which was not the case at baseline suggesting maintenance of ventilation and improvement in gas exchange in the AF group. This is promising given that reduced baseline HVP and RBC:Barrier are both associated with disease progression3,4 and can potentially monitor response in an individual patient8. Although not statistically significant, HVP seems to be relatively maintained in the AF group and decreasing in the no-AF group, while RBC:Barrier increased (improved) in the AF group and remained relatively consistent without AF medication (Figure 1, Table 1). This is a notable difference in apparent treatment response between ventilation and gas-exchange that encourages further investigation. While the change in HVP over time within each individual subject was not significantly different across medication groups, the change in RBC:Barrier was significant (Figure 1). In fact, RBC:Barrier increased over 1 year in all 8 patients taking AF medication and decreased in all but 1 of the 6 no-AF patients.Conclusion
Imaging biomarkers of ventilation and gas exchange measured using HP 129Xe MRI were associated with AF treatment of IPF (Pirfenidone and Nintedanib). There were no longitudinal associations found between AF treatment and spirometry. Significant differences were found in HVP and RBC:Barrier ratio between medication groups after 1 year that were not present at baseline. We also found that the RBC:Barrier ratio significantly increased in IPF patients taking AF medication after 1 year, indicating better gas diffusion in the lung at the microstructural level. These results suggest that the HVP and RBC:Barrier ratio may be useful biomarkers of treatment response in IPF.Acknowledgements
We would like to acknowledge research support from GE Healthcare. Additional funding for this work was provided by the University of Wisconsin, Department of Radiology and Medical Physics, Research and Development Fund, WARF, Pulmonary Imaging Center NIH/ORIP S10 OD016394, NIH/NHLBI R01HL126771 and R01 EB021314.References
1) Lee JW, et al. Clinical findings and outcomes in patients with possible usual interstitial pneumonia. Respir Med. 2015; 109(4):510–6
2) Mammarappallil JG, et al. New Developments in Imaging Idiopathic Pulmonary Fibrosis With Hyperpolarized Xenon Magnetic Resonance Imaging. J Thoracic Imaging 2019; 34(2):136-150
3) Hahn AD, et al. Functional MRI of Regional Gas Exchange in IPF Disease Progression. Am J Respir Crit Care Med 2020;201:A5995
4) Carey KJ, et al. Quantitative Magnetic Resonance Imaging and Computed Tomography Measures of Progression in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2020;201:A4510
5) Vancheri C, et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis: Results of the INJOURNEY Trial. Am J Respir Crit Care Med 2018; 197(3):356-363
6) Zha W, et al. Regional Heterogeneity of Lobar Ventilation in Asthma Using Hyperpolarized Helium-3 MRI. Acad Radiol 2018; 25(2):169-178
7) Kaushik SS, et al. Probing the regional distribution of pulmonary gas exchange through single-breath gas- and dissolved-phase 129Xe MR imaging. J. Appl. Physiol. 2013; 115:850–860.
8) Mummy DG, et al. Ventilation defects on hyperpolarized helium-3 MRI in asthma are predictive of 2-year exacerbation frequency. J Allergy Clin Immunol. 2020; 146(4):831-839.e6
Figures