0583
Decreased muscular perfusion in dermatomyositis: initial results detected by Inflow-based vascular-space-occupancy MR imaging1Department of Medical Imaging, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Neurosection, Division of MRI Research, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Philips healthcare, Guangzhou, China
Synopsis
Dermatomyositis (DM) is a chronic autoimmune microangiopathy. Accurate quantification of muscular microcirculation can assist in early diagnosis and improve clinical outcomes. Inflow-based vascular-space-occupancy (iVASO) is a novel perfusion technique without the need for exogenous contrast agents. This study aimed to determine the potential diagnostic value of iVASO-MRI for patients with DM. The results showed decreased arteriolar muscular blood volume (MBVa) in DM patients, which worsens with the progression of disease, and the diminished MBVa in morphologically-normal appearing muscles. This suggests that iVASO-derived MBVa has the potential to be used as a biomarker for early diagnosis of DM.
INTRODUCTION
Dermatomyositis (DM) is a chronic autoimmune microangiopathy. Structural MRI plays an important role in the diagnosis of DM, monitoring of disease progression and therapeutic responses, and the guidance of biopsy. However, it cannot provide quantitative information on microvascular changes1. Impaired skeletal muscle microcirculation occurs prior to myasthenia and morphological abnormalities of skeletal muscles2, 3. Therefore, accurate quantification of muscular microcirculation may contribute to the early diagnosis and treatment of DM, and effectively prevent irreversible muscle damage and improve clinical outcomes4. Inflow-based vascular-space-occupancy (iVASO) is a novel perfusion technique without the need for exogenous contrast agents5. This study aimed to determine the diagnostic value of iVASO-MRI for patients with DM.METHODS
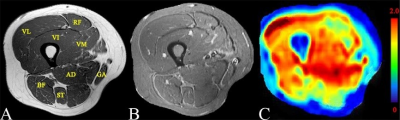
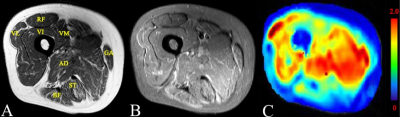
Twenty-five patients with DM and 22 healthy volunteers underwent structural MRI and 3D-iVASO scan with a 3T clinical scanner (Achieva TX, Philips). 3D-iVASO was performed with gradient spin echo readout, TE = 10 ms; TR/TI = 5000/1040, 3100/862, 2500/756, 2000/641, 1700/558, 1300/430 ms; voxel = 2.5 × 2.5 × 6 mm3, 14 slices; parallel imaging acceleration (SENSE) = 2 × 2; crusher gradients of b = 0.3 s/mm2 and Venc = 10 cm/s on z-direction. Maximum arteriolar muscular blood volume (MBVa_max) and mean MBVa (MBVa_mean) of four subgroups of muscles (normal muscles, morphologically-normal appearing [MN] muscles, edematous muscles, and atrophic or fat-infiltrated [AF] muscles) were obtained. MBVa_max and MBVa_mean among the different subgroups were compared, and repeat testing was performed in 20 subjects for assessment of reproducibility.RESULTS
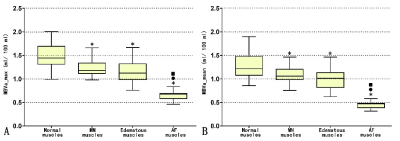
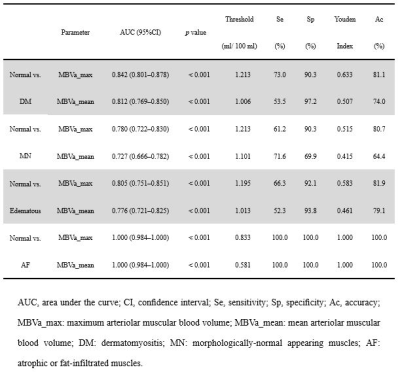
Compared to normal muscles in normal subjects, MN muscles, edematous muscles, and AF muscles in DM patients showed a significant decrease of both MBVa max and MBVa_mean (p < 0.001) (Figures 1, 2 and 3). Both parameters of AF muscles were significantly lower than MN and edematous muscles (p < 0.001). Areas under receiver operating characteristic curve were 0.842 and 0.812 for MBVa_max and MBVa_mean respectively, in discriminating DM patients from normal volunteers (Table 1). As a measure of test-retest studies, the ICC was 0.990 (95% confidence interval [CI]: 0.986 to 0.993) and 0.990 (95% CI: 0.987 to 0.993) for MBVa_max and MBVa_mean, respectively.DISCUSSION
We found that quantitative perfusion using iVASO could be reproducibly obtained in both normal subjects and patients with DM. More importantly, we found that patients with DM had significantly lower arteriolar blood volume. In particular, normal-appearing muscles shown on structural MRI exhibited impaired perfusion, and atrophic or fat-infiltrated muscles showed more severe reduction in arteriolar blood volume than did edematous muscles. Recently, some scholars claimed that the primary cause of muscle ischemia in DM was not capillary loss but the whole microvascular unit damage secondary to the destroyed arterioles (upstream of the capillaries)6. Emslie-Smith et al. used the Ulex europaeus agglutinin I method (UEA-I) to visualize microvasculature and quantitatively analyzed capillary density in DM patients, and found a significant decline of microvasculature in the early stage of disease3. Another group found that the metabolic abnormality of muscles in DM patients was secondary to impaired blood supply in a study with phosphorus MR spectroscopy (31P-MRS)7. However, these radiologic studies were limited to the analysis of capillaries, and did not include an assessment of arteriolar compartment. iVASO used in this study primarily assessed arteriolar blood volume changes in muscle tissue. Our data reveal decreased MBVa in patients with DM compared to normal controls, which is in line with conclusions from the above-stated studies. Interestingly, this study showed decreased arteriolar perfusion in morphologically-normal appearing muscles, i.e., normal appearing muscles on structural MRI. This indicated that iVASO may be more sensitive to inflammatory myopathies. Tomasova et al. found that the so-called morphologically-normal appearing muscles were not completely normal but had a certain level of inflammation that did not reach a degree sufficient to be visible on structural MRI8. Metabolic abnormalities have been observed in these types of muscles using MRS9, which lends support to our results. However, studies with diffusion tensor imaging did not show a significant difference between the morphologically-normal appearing muscles and normal muscles10. This may also indicate that perfusion disturbances occur earlier than inflammatory cell infiltration and other micro-environmental changes. Therefore, MBVa has the potential to be a useful early imaging marker for myositis, and aid in clinical decision-making.CONCLUSION
iVASO can reproducibly quantify thigh muscular arteriolar blood volume and is able to discriminate between normal volunteers and patients with DM.Acknowledgements
No acknowledgement found.References
1. Maurer B, Walker UA. Role of MRI in diagnosis and management of idiopathic inflammatory myopathies. Curr Rheum Rep. 2015; 17(11):67.
2. Mammen AL. Dermatomyositis and polymyositis: Clinical presentation, autoantibodies, and pathogenesis. Ann N Y Acad Sci. 2010; 1184:134-153.
3. Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990; 27(4):343-356.
4. Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015; 372(18):1734-1747.
5. Hua J, Qin Q, Pekar JJ, et al. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR BioMed. 2011; 24(10):1313-1325
6. Gitiaux C, Kostallari E, Lafuste P, et al. Whole microvascular unit deletions in dermatomyositis. Ann Rheum Dis. 2013; 72(3):445-452.
7. Cea G, Bendahan D, Manners D, et al. Reduced oxidative phosphorylation and proton efflux suggest reduced capillary blood supply in skeletal muscle of patients with dermatomyositis and polymyositis: a quantitative 31P-magnetic resonance spectroscopy and MRI study. Brain. 2002; 125(Pt 7):1635-1645.
8. Tomasova SJ, Charvat F, Jarosova K, et al. The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology (Oxford). 2007; 46(7):1174-1179.
9. Subhawong TK, Wang X, Machado AJ, et al. 1H Magnetic resonance spectroscopy findings in idiopathic inflammatory myopathies at 3 T: feasibility and first results. Invest Radiol. 2013; 48(7):509-516.
10. Ai T, Yu K, Gao L, et al. Diffusion tensor imaging in evaluation of thigh muscles in patients with polymyositis and dermatomyositis. Br J Radiol. 2014; 87(1043):20140261.
Figures